Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ingeniería e Investigación
Print version ISSN 0120-5609
Ing. Investig. vol.36 no.2 Bogotá May/Aug. 2016
https://doi.org/10.15446/ing.investig.v36n2.52369
DOI: http://dx.doi.org/10.15446/ing.investig.v36n2.52369
Colombian fusel oil
El aceite de fusel de Colombia
N. Montoya1, J. Durán2, F. Córdoba3, I. Gil4, C. Trujillo5, and G. Rodríguez6,1*
1 Chemical engineer. Affiliation: PhD student, Universitiy of Alberta. Edmonton, Alberta, Canadá. E-mail: nmontoya@ualberta.ca
2 MSc. Chemical Engineering. Affiliation: PhD student, University of Calgary. Calgary, Canadá. E-mail: jaduran@ucalgary.ca
3 MSc. Chemical Engineering Student. Affiliation: MSc student, Universidad Nacional de Colombia. Colombia. E-mail: fpcordobav@unal.edu.co
4 Ph.D. Université de Lorraine. Affiliation: Assistant Professor, Universidad Nacional de Colombia. Colombia. E-mail: idgilc@unal.edu.co
5 Ph.D. Universidad Nacional de Colombia. Affiliation: Full Professor, Universidad Nacional de Colombia. Colombia. E-mail: catrujillo@unal.edu.co
6 Ph.D. Universidad Nacional de Colombia. Affiliation: Associate Professor, Universidad Nacional de Colombia. Colombia. E-mail: grodriguezn@unal.edu.co
1* by chromaticity features using self-organizing, E-mail: grodriguezn@unal.edu.co, Tel: + 57 1 3165000 Ext. 13 553, Fax: +57 1 3165617
How to cite: Montoya, N., Durán, J., Córdoba, F., Gil, I., Trujillo, C., & Rodríguez, G. (2016). Colombian fusel oil. Ingeniería e Investigación, 36(2), 21-27. DOI: 10.15446/ing.investig.v36n2.52369.
ABSTRACT
By-products valorization in bio-fuels industry is an important issue for making the global process more efficient, more profitable and closer to the concept of biorefinery. Fusel oil is a by-product of bioethanol production that can be considered as an inexpensive and renewable raw material for manufacturing value-added products. In this work, results in terms of composition and physicochemical properties of six samples of fusel oil from industrial alcohol facilities are presented. Composition of the main components was established by gas chromatography. Complementary techniques, such as headspace solid-phase microextraction and gas chromatography-mass spectrometry (GC-MS), were used for detection of minor components. Fifty-five compounds were identified. Physicochemical properties such as density, acid value, moisture content and true boiling point curves were determined. Results are useful in the conceptual design of separation strategies for recovering higher alcohols, as well as to consider new options of valorization alternatives for fusel oil.
Keywords: Fuel ethanol, higher alcohols, GC-MS analysis, solid-phase microextraction, TBP.
RESUMEN
Los subproductos para valorización en la industria de los biocombustibles es un tema importante para alcanzar un proceso global más eficiente, más rentable y más cerca del concepto de biorrefinería. El aceite de fusel es un subproducto de la producción de bioetanol que se puede considerar como una materia prima barata y renovable para la fabricación de productos de valor agregado. En este trabajo se presentan los resultados en términos de composición y de las propiedades físico-químicas de seis muestras de aceite de fusel de las instalaciones industriales de alcohol. La composición de los componentes principales se estableció mediante cromatografía de gases. Técnicas complementarias, como espacio de cabeza, microextracción en fase sólida, y cromatografía de gases-espectrometría de masas (GC-MS), fueron utilizadas para la detección de componentes menores. Se identificaron cincuenta y cinco compuestos. Se determinaron las propiedades físico-químicas, como la densidad, el índice de acidez, el contenido de humedad y las curvas de puntos verdaderos de ebullición. Los resultados son útiles en el diseño conceptual de las estrategias de separación para la recuperación de alcoholes superiores, así como para comprobar las opciones de alternativas de valorización del aceite de fusel.
Palabras Clave: Etanol-combustible, alcoholes pesados, análisis GC-MS, micro-extracción en fase sólida, TBP.
Received: February 29th 2016 Accepted: July 26th 2016
Introduction
Fusel oil is a by-product of biomass fermentation in the industrial bioethanol production. It is a mixture of higher alcohols, mainly composed by i-amyl and other short-chain alcohols. The bioethanol industry is rapidly growing, and so is fusel oil production. Also, in countries where bioethanol is produced at a large scale, by-product utilization has become in an important issue in making the global process less polluting and more profitable. Fusel oil has been considered as a low-value material that some ethanol producers burn to produce energy. However, it can be used as a cheap and renewable source for the production of biosolvents, extractants, flavoring agents, medicinal and plasticizers (Guvenc et al., 2007).
The quality and quantity of fusel oil generated during alcohol production depends on the type and method of preparation of the mash used for fermentation, nitrogenous substances added, yeast, conditions under which fermentation proceeds, and on the method of removal of fusel oil (Patil et al., 2002). Likewise, the yield of fusel oil obtained in a commercial plant may vary between one and eleven liters per each thousand liters of alcohol produced (absolute basis) (Patil et al., 2002).
Some metabolites involved in fermentation processes have been identified as source of higher alcohols. Ehrlich (1907) and Klosowski et al. (2010) have attributed the production of higher alcohols to amine nitrogen assimilation. Considering the high content of amyl alcohols in fusel oil (Maiorella et al., 1981), it is important to mention the role played by leucine and isoleucine as source molecules of 3-methyl-1-butanol and 2-methyl-1-butanol, respectively (Ribereau-Gayon et al., 2006). Roehr (2001) indicates that fusel oil is formed from a-keto acids, derived from aminoacids. Pfenninger (1963) and Roehr (2001) compared fusel oil composition based on the feedstock for fermentation. Their results indicate that butanol and i-amyl alcohols content increases when molasses and fruits are the raw material for fermentation. However, those authors point out the importance of pH in the fermenter in the production of higher alcohols.
Fusel oil is used as a raw material for the production of amyl and butyl alcohols which have some different but significant applications: The work of Ogonowski and Sikora (2000) deals with catalytic conversion of methanol and i-butanol into ethers. Similarly, Klier et al. (1997) studied the conversion of higher alcohols into different ethers over multifunctional catalysts. Vaze et al. (1997) presented an alternative of valorization consisting on the electrochemical oxidation of alcohols to produce carboxylic acids. Mitra et al. (1997) studied the alkylation of aromatic compounds with alcohols such as butanol and i-butanol in order to provide green feedstocks to the pharmaceutical industry. Garcia et al. (1997) and Liaw et al. (1998) also offer alternatives for using butanol and i-amyl alcohol in pharmaceutical applications.
Moreover, in the last decade, alcohols from fusel oil have been used for the manufacture of bioproducts with the advantage of being environmentally safe, renewable, and in some cases biodegradable. Ozgulsun et al. (2000) studied the esterification reaction of oleic acid with a fraction of fusel oil from molasses to produce lubricating oil. Dormo et al. (2004) synthesized a biolubricant from fusel oil by enzymatic esterification. Guvenc et al. (2007) and Bandres et al. (2010) performed the syntheses of biobased solvents to obtain acetates, carbonates and i-valerates with i-amyl alcohol from fusel oil.
Some studies related to the characterization of fusel oil have been published. The first ones were developed in the first half of the 20th century. Penniman et al. (1937), through colorimetric methods, Schicktanz et al. (1939) using azeotropic distillation, and Ikeda et al. (1956) from chromatographic analysis, were able to identify the main alcohols (C2 to C5) present in fusel oil from different sources. Marvel and Hager (1924) and Webb et al. (1952) found esters derived from short-chain alcohols, mainly i-amyl alcohol, and some carboxylic acids (C10 to C20). Results from comprehensive studies developed with improved analytic techniques, such as mass spectrometry (MS), high resolution gaseous chromatography (HRGC), headspace solid phase microextraction (SPME), among others, have shown a higher amount of components in different fractions of fusel oil. For instance, Patil et al. (2002) exposes the characterization of fusel oil in terms of two main fractions: a low boiling fraction (LBF) and a high boiling fraction (HBF). The first one corresponds to 95-98% v/v and is mainly i-amyl alcohol. The second one is composed by fatty acids and their esters, also some pyrazines and unsaponificable material. In addition, Garcia (2008) reports the presence of components with commercial value, such as farnesol and nerolidol.
This paper focuses on the characterization of six samples of fusel oil from Colombian biofuel and beverage industries. Their composition was studied by GC, SPME and GC-MS, and their physicochemical properties, such as density, acid value, water content and true boiling point curves were also determined. This information can be used to design separation processes of higher alcohols, to develop new products and to evaluate alternatives for valorization of fusel oil.
Materials and methods
Reagents
Six samples of fusel oil obtained from Colombian bioethanol (F1 to F4) and beverage (F5 to F6) industries were analyzed. All reagents used for chromatographic analyses were analytical grade. Ethanol, n-propanol, i-propanol, n-butanol, i-butanol, and i-amyl alcohol were supplied by Merck KGaA. n-Amyl alcohol was purchased from Scharlau Chemie S.A. The internal standard used in this study was methyl-i-propyl-ketone (MIPK) provided by Merck KGaA. Methanol obtained from EMD Chemicals S.A. was used as solvent. Karl-Fischer reagent was supplied by J.T. Baker Inc.
True boiling point (TBP) curves
TBP curves were made in a batch distillation still at 75,06 kPa, using the ASTM D86 procedure. Data were corrected at 101. kPa as suggested in the standard method mentioned above. The estimated uncertainty in the temperature was 0,1 K, and the uncertainty in volume measurements was 0,05 mL.
Moisture content
Measurements were performed according to ASTM E203 using a Mettler Toledo DL53 automatic titrator with an electrode DM 142.
Chromatographic analysis
The chromatographic technique was developed as suggested by Grob and Barry (2004) and GC suppliers (Hewlett-Packard Co., 1988, Restek Corp., 2003, Varian Inc., 2004). The analyses were performed on a Perkin-Elmer Clarus 500 apparatus equipped with an automatic injector, an Elite Plot-Q column (30 m x 0,53 mm i.d. x 1 mm film), and flame ionization detector (FID at 270°C). The initial oven temperature was programmed at 150°C for 1 min, with a heating ramp of 3°C/min, raising to 220°C and then kept for 5 min. The injection temperature was 250°C and the split injection mode was adjusted to 70:1 ratio. Helium at 31,5 cmN/s was used as carrier.
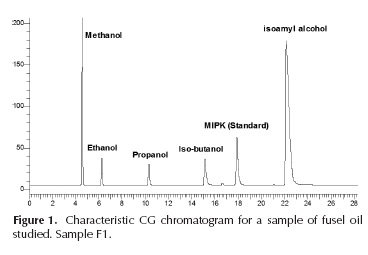
The purity of alcohols used as calibration standards was taken into account to correct the obtained curves. N,N-dimethylformamide, diethyl ketone and methyl i-butyl ketone were tested as internal standard candidates. Methyl i-propyl ketone (MPIK) was selected for its retention time and high response factor (defined as area unit per mass unit). Initial analyses showed absence of methanol; therefore, it was chosen as solvent. Calibration standards were prepared as recommended by ASTM D4307. Fourteen standards of each alcohol were prepared gravimetrically with the addition of 80 mg of internal standard in each vial. Ethanol, 1-propanol, i-propanol, n-butanol, i-butanol, t-butanol, i-amyl and n-amyl alcohols were used to prepare calibration curves. Only n-pentanol peak presented tailing in the analyzed patterns. t-Butanol and n-pentanol were not detected in the samples under study. Acetaldehyde and acetic acid were identified but were not quantified because of their low response factor. Figure 1 shows a characteristic GC chromatogram of studied fusel oil samples.
Solid phase micro-extraction (SPME) procedures
The method used for the SPME was a modification from the one reported by Nonato et al. (2001). For this study, a fiber (85 mm, polyacrylate) was conditioned in the GC injector port at 260°C for 2 hours prior to use - as the supplier recommended. Samples of fusel oil were transferred to 10 mL vials capped with PTFE-faced butyl rubber seals. Extractions in the headspace mode were carried out at 30°C for 10 min. After the extractions, the fiber was introduced immediately into the GC-MS chromatograph injector port for 3 minutes in split mode (15:1). The analytes were thermally desorbed at 260°C and subsequent chromatographic analysis was performed.
Mass spectrometry procedure
GC-MS analysis was a modification from the method reported by Nonato et al. (2001). A Shimadzu GCMS QP 2010S series equipped with a SGE BP-20 column (30 m x 0,32 mm i.d. x 0,25 mm film) was used. Peak identification was performed using a quadrupole mass-spectrometer Shimadzu MS-QP-2010, directly attached to the GC system. The initial oven temperature was programmed at 40°C for 5 min, raised to 100°C at 5°C/min, then at 10°C/min to 240°C and held there for 20 min. Injection was made as mentioned in the SPME procedures and the carrier was helium at 31,5 cmN/s. Also, direct injection of the liquid phase was made to detect components with low vapor pressure. In this case, samples were diluted with dichloromethane in a ratio of 1:16. The detector was turned on only after six minutes of running, and turned off between nine to ten minutes forty five seconds to avoid detector saturation with water and alcohols in higher proportion (C2-C6).
Other assays
The samples' density was determined with a digital densimeter Mettler Toledo 30PX. Each measurement was performed by triplicate at 20 °C ± 1 °C. Acid value determination was made by potentiometric titration with a 0,12 M potassium hydroxide solution in azeotropic ethanol. An automatic potentiometric titrator Mettler Toledo DL53, with an electrode DG113-SC, was used for the tests. Each test was done by triplicate.
Results and discussion
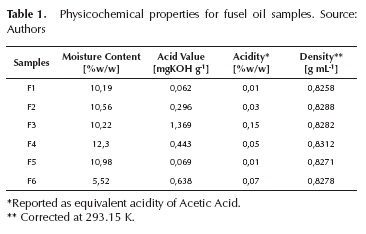
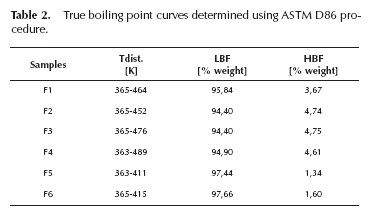
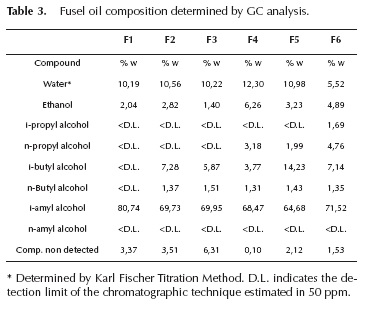
Fusel oil samples were characterized by their composition and physicochemical properties. Results are presented in Table 1 for the physicochemical properties, Table 2 for the true boiling point curves and Table 3 for the composition of the main components.
Densities of the samples are higher than the density of i-amyl alcohol (0,810 g/mL) at the same temperature, which is expected due to the presence of water and higher molecular weight compounds. For instance, F4 density is the highest among samples following its high water content (12,3% of its weight). Water content in fusel oil is dependent on the production processes. Some plants wash fusel with water in order to strip the residual ethanol, leaving fusel saturated with water. Sample F6 has the lowest water content; a value that suggests this sample was not washed. The water content agrees with the relative high ethanol content in this sample. F1 has the lowest density and consequently, the highest content of i-amyl alcohol. In samples F2, F3, F4 and F6, the composition distribution is broader; their properties are affected by the presence of propyl and butyl alcohols. The acid value does not follow any particular tendency and results vary between samples by at least one order of magnitude. This number is an indicative of the amount of fatty acids and therefore heavy esters.
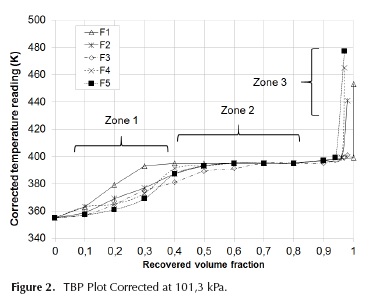
True boiling point curves analyses were applied in order to establish the general behavior of the composition distribution in the samples. In Figure 2, TBP curves are shown. The distillation temperatures are also collected in Table 2 with the different boiling temperatures for the 5 samples.
As indicated in Figure 2, TBP curves were divided into three zones. Zone 1 shows the distillation region of water, ethanol, n-propanol, i-propanol, n-butanol and i-butanol. The length of zone 2 indicates a direct proportionality to i-amyl alcohol content in samples, which is verified by checking Table 3. Finally, zone 3 corresponds to the distillation region of the heaviest components.
The beginning of the plateau where i-amyl alcohol distillates is an indicative of the content of water and low molecular weight alcohols, thus TBP curves of samples F2 and F5 have a small slope below 383 K. Zone 1 is prolonged to 50% volume, which correlates well with the high content of propanol and butanol of these samples, as shown in Table 3. First drop distillation temperature is strongly influenced by ethanol content. Figure 2 shows that F3 TBP curve starts around 366 K, which is an evidence of its low ethanol content. Similarly, F5 distillation curve starts at the lowest temperature (around 364 K), and keeps its low distillation temperatures until 70% of the recovered volume, showing the most extended zone 1. This indicates that F5 contains a high percentage of water and C2 to C4 alcohols, as can be seen on Table 3. Finally, F6 presents a high slope in zone 1, indicating low water and ethanol content.
Regarding zone 2, the TBP curve of sample F1 has the largest length (between 30 °% vol. to 90 °% vol.) because of its high i-amyl alcohol content. Sample F5 has the shortest second zone, so it can be inferred this sample has the lowest content of i-amyl alcohol, confirming the data on Table 3.
The distillation final temperature is an indicative of the molar mass of the heaviest compounds. Samples F5 and F6, obtained from beverage industries, show the lowest final temperatures. Samples F1 to F4 (from sugar mills) have the maximum distillation temperatures, above 411 K, which indicate that the chemical identity of their heavy components is different. Zone 3 is the smallest of the three, being shorter for samples from the sugar mills and larger for the samples obtained from the beverage industry.
The chromatographic technique used ensures separation of eluted compounds for at least 15 seconds (Figure 1). Calibration curves from the chromatographic analysis were correlated with first order equations, obtaining correlation factors above 0,991 in all cases. Those curves were verified against synthetic mixtures of alcohols with known composition. Maximum deviation was determined as 0,14% in mass fraction. Detection limit was established as a half of minimum calibration standard concentration. In all cases this limit was between 25 and 75 mass ppm. All calibration curves were covering a range between the detection limit and 99,0% in mass.
As shown in Table 3, composition of C3 - C5 alcohols presents some variations among samples. Sample F1 is composed by water, ethanol and i-amyl alcohol; while samples F2 and F3 also have C4 alcohols. F4, F5 and F6 have all these plus C3 alcohols.
Changes in composition of fusel oil are due to many factors: those that potentiate the apparition of fusel oil are the lack of nitrogen, high temperatures, large times of fermentation, high levels of amino acids, high sugar adjunct levels, higher than normal yeast rates and longer time interval between fermentation and distillation. On the other hand, factors that reduce the production of fusel oil are large capacity of fermenters, high ethanol yield and optimum temperature control for the specific yeast strain, letting it give higher selectivity to ethanol. Related to the presence or absence of some alcohols in the samples, it is important to mention that this depends on the aminoacids available during fermentation. Thus, isoleucine and valine both lead to 2-methyl-1-butanol, valine leads to 3-methyl- 1- butanol, threonine to 1-propanol, and 2-phenylalanine to 2- phenylethanol. Also, the treatment given to fusel oil in the facilities can vary its composition. For instance, a common treatment is to wash the fusel oil with water to separate soluble components, typically found in low concentrations.
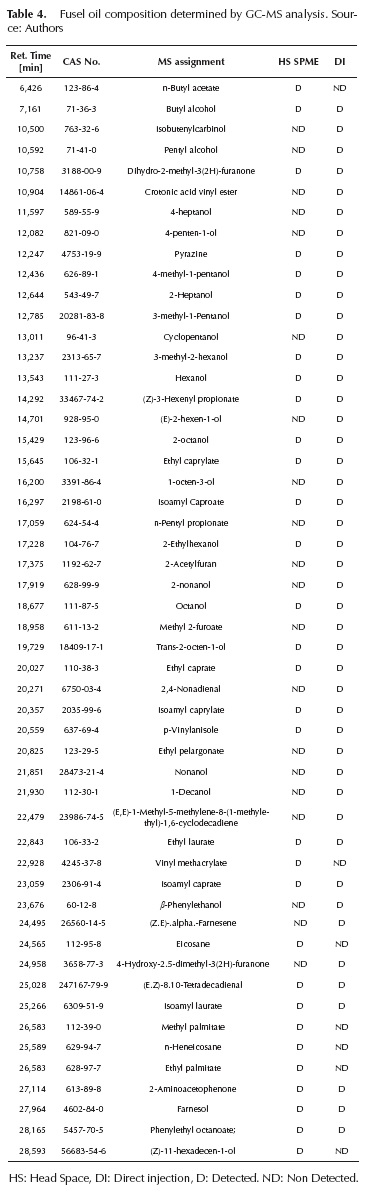
Table 3 shows that there are a percentage of non-identified compounds (among 0,1% and 6% of the weight) because of the limitations of the chromatographic technique. A further analysis was necessary and GC-MS was used for that purpose. Sample F1 was analyzed by GC-MS chromatography; the results are summarized in Table 4 with the different components identified by means of mass spectrometry coupled with gas chromatography.
Fifty-one compounds were identified in the oil sample. Forty-nine of these had not been identified by the previous GC chromatography analysis. Related to the main compounds detected, alcohols and esters represent the largest groups, with seventeen and sixteen individual compounds identified, respectively. Alcohols with wideranging structural differences (unsaturation, branching, cyclization, among others) were found. Most of the esters detected correspond to ethyl and i-amyl compounds, formed as a product of yeast metabolism or by esterification of fatty acids in the presence of ethanol and i-amyl alcohol at high concentration. Minor proportion compounds such as alkanes, aldehydes, amines, ethers, ketones, lactones and sesquiterpenes were also found.
Conclusions
The characterization of six samples of fusel oil from industrial alcohol facilities in Colombia shows that i-amyl alcohol is the main constituent of all samples. Besides i-amyl alcohol, short chain alcohols, water and high boiling point compounds are also found. Composition is affected by the origin of the samples, being richer in heavy compounds those coming from sugar mills. Identification and quantification of main components by GC analyses was achieved. Further analyses by headspace solid-phase microextraction and GC-MS allowed identification of minor constituents, volatile and heavy compounds.
Acknowledgements
The authors thank Universidad Nacional de Colombia – Sede Bogotá, where this study was made, and the financial support provided by Ecopetrol S. A. and Colciencias through the project number 110149026038.
References
Bandres, M., de Caro, P., Thiebaud-Roux, S., & Borredon, M. (2010). Green syntheses of biobased solvents. C. R. Chim., Vol. 14, No. 7-8, 636-646. DOI: 10.1016/j.crci.2010.07.008. [ Links ]
Dörmo, N., Bélafi-Bakó, K., Bartha, L., Ehrenstein, U., & Gubicza, L. (2004). Manufacture of an environmental-safe biolubricant from fusel oil by enzymatic esterification in solvent-free system. Biochem. Eng. J., Vol. 21, No. 3, 229 - 234. DOI: 10.1016/j.bej.2004.06.011. [ Links ]
Ehrlich, F. (1907). Über die Bedingungen der Fuseölbildung und über ihren Zusammenhang mit dem Eiweissaufbau der Hefe. Berichte Deutsch Chem. Gesellschaft, Vol. 40, No. 1, 1027-1047. DOI: 10.1002/cber. 190704001156. [ Links ]
Garcia, M.A., Aramayona, J.J., Bregante, M.A., Fraile, L.J., & Solans, C. (1997). Simultaneous determination of verapamil and norverapamil in biological samples by high-performance liquid chromatography using ultraviolet detection. J. Chromatogr. B, Vol. 693, No. 2, 377-382. DOI: 10.1016/S0378-4347(97)00058-3. [ Links ]
Garcia, V. (2008). Subproduto de destilaria de óleo fúsel: caracterização da composição química e estudo de sua aplicação industrial (Mestre em Engenharia de Processos Químicos e Bioquímicos). Centro Universitário do Instituto Mauá de Tecnologia, São Caetano do Sul: Brasil. Recuperado de http://maua.br/files/dissertacoes/subproduto-de-destilaria-de-oleo-fusel.pdf. [ Links ]
Güvenç, A., Kapucu, N., Kapucu, H., Aydogan, O., & Mehmetoglu, Ü. (2007). Enzymatic esterification of isoamyl alcohol obtained from fusel oil: Optimization by response surface methodology. Enzyme Microb. Technol., Vol. 40, No. 4, 778-785. DOI: 10.1016/j.enzmictec.2006.06.010. [ Links ]
Grob, R., & Barry, M. (2004). Modern Practice in Gas Chromatography. Fourth Edition. United States of America: Wiley and Sons Publications. DOI: 10.1002/0471651141. [ Links ]
Hewlett-Packard Co. (1988). Analytical Supplies Catalog and Catalog Reference Guide. West Germany: Hewlett-Packard Co. [ Links ]
Ikeda, R.M., Webb, A.D., & Kepner, R.E. (1956). Comparative analysis of fusel oils from Thompson Seedless, Emperor, and Muscat of Alexandria Wines. J. Agric. Food Chem., Vol. 4, No. 4, 355-363. DOI: 10.1021/jf60062a009. [ Links ]
Klier, K., Beretta, A., Sun, Q., Feeley, O.C., & Herman, R.G. (1997). Catalytic synthesis of methanol, higher alcohols and ethers. Catal. Today, Vol. 36, No. 1, 3-14. DOI: 10.1016/S0920-5861(96)00190-3. [ Links ]
Klosowski, G., Mikulski, D., Grajewski, J., & Blajet-Kosicka, A. (2010). The influence of raw material contamination with mycotoxins on alcoholic fermentation indicator. Bioresour. Technol., Vol. 101, No. 9, 3147-3152. DOI: 10.1016/j.biortech.2009.12.040. [ Links ]
Liaw, W.J., Ho, S.T., Wang, J.J., Hu, O.Y.P., & Li, J.H. (1998). Determination of morphine by high-performance liquid chromatography with electrochemical detection: application to human and rabbit pharmacokinetic studies. J. Chromatogr. B, Vol. 714, No. 2, 237-245. DOI: 10.1016/S0378-4347(98)00230-8. [ Links ]
Maiorella, B., Wilke, Ch.R., & Blanch, H.W. (1981). Alcohol production and recovery. Adv. Biochem. Eng. Biotechnol., Vol. 20, 43-92. DOI: 10.1007/3-540-11018-6_3. [ Links ]
Marvel, C.S., & Hager, F.D. (1924). Bauer Oil, The High-Boiling Residue From Molasses Fusel Oil. J. Am. Chem. Soc., Vol. 46, No. 3, 726-731. DOI: 10.1021/ja01668a024. [ Links ]
Mitra, A., Subramanian, S., Das, D., Satyanarayana, V.V.C., & Chakrabarty, D.K. (1997). Alkylation of aromatics on zeolite beta. Unusual butylation of benzene with isobutanol. Appl. Catai. A, Vol. 153, No. 1-2, 233-241. DOI: 10.1016/ S0926-860X(96)00321-3. [ Links ]
Nonato, E.A., Carazza, F., Silva, F.C., Ciomara, R., & Cardeal, Z. (2001). A Headspace Solid-Phase Microextraction Method for the Determination of Some Secondary Compounds of Brazilian Sugar Cane Spirits by Gas Chromatography. J. Agric. Food Chem., Vol. 49, No. 8, 3533-3539. DOI: 10.1021/jf000896r. [ Links ]
Ogonowski, J., & Sikora, E. (2000). Coupling of methanol and isobutanol to ethers over y-alumina catalyst. Kinetic Study. React. Kinet. Catal. Lett., Vol. 70, No. 2, 235-241. DOI: 10.1023/A:1010320312175. [ Links ]
Özgülsün, A., Karaosmanoglu, F., & Tüter, M. (2000). Esterification reaction of oleic acid with a fusel oil fraction for production of lubricating oil. J. Am. Oil Chem. Soc., Vol. 77, No. 1, 105-109. DOI: 10.1007/s11746-000-0017-5. [ Links ]
Patil, A.G., Koolwal, S.M., & Butala, H.D. (2002). Fusel Oil: Composition, removal and potential utilization. Int. Sugar J., Vol. 104, 51-58. [ Links ]
Penniman, W.B.D., Smith, D.C., & Lawshe, E.I. (1937). Determination of Higher Alcohols in Distilled Liquors. Ind. Eng. Chem. Anal. Ed., Vol. 9, No. 2, 91-95. DOI: 10.1021/ac50106a016. [ Links ]
Pfenninger, H. (1963). Gaschromatographische Untersuchungen von Fuselölen aus verschiedenen Gäsprodukten. Z. Lebensm. Unters. Forsch., Vol. 119, 401-415. DOI: 10.1007/BF01300142. [ Links ]
Restek Corp. (2003). Chromatography Products 2003/2004, Restek Corporation Inc.: United States of America. [ Links ]
Ribereau-Gayon, P., Glories, Y., Maujean, A., & Dubourdieu, D. (2006). Handbook of Enology. The Chemistry of Wine, Vol. 2, John Wiley & Sons Ltd.: Paris. [ Links ]
Roehr, M. (2001). The biotechnology of ethanol. Classical and future applications, Wiley-VCH Verlag GmbH: Weinheim. [ Links ]
Schicktanz, S.T., Etienne, A.D., & Steele, W.I. (1939). Analysis of Fusel Oil by Azeotropic Distillation. Ind. Eng. Chem. Anal. Ed., Vol. 11, No. 8, 420-422. DOI: 10.1021/ac50136a003. [ Links ]
Van Gheluwe, G., Chen, E., & Valyi, Z. (1976). Factors Affecting the Formation of Fusel Alcohols during Fermentation. MBAA Technical Quarterly., Vol. 12, No. 3, 169-175. [ Links ]
Varian Inc. (2004). GC 3380 Documentation: Quick Reference Guide, Varian GC Getting Started & Operator's Manual, Varian Incorporated: United States of America. [ Links ]
Vaze, A.S., Sawant, S.B., & Pangarkar, V.G. (1997). Electrochemical oxidation of isobutanol to isobutyric acid at nickel oxide electrode: improvement of the anode stability. J. Appl. Electrochem., Vol. 27, No. 7, 584-588. DOI: 10.1023/A:1018406914144. [ Links ]
Webb, A.D., Kepner, R.E., & Ikeda, R.M. (1952). Composition of Typical Grape Brandy Fusel Oil. Anal. Chem., Vol. 24, No. 12, 1944-1949. DOI: 10.1021/ac60072a020. [ Links ]