Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Ingeniería e Investigación
Print version ISSN 0120-5609
Ing. Investig. vol.37 no.1 Bogotá Jan./Apr. 2017
https://doi.org/10.15446/ing.investig.v37n1.57991
DOI: http://dx.doi.org/10.15446/ing.investig.v37n1.57991
Hydrodeoxygenation of furfuryl alcohol over Cu/MgAl and Cu/ZnAl catalysts derived from hydrotalcite-like precursors
Hidrodesoxigenación de furfuril alcohol sobre catalizadores de Cu/MgAl y Cu/ZnAl derivados de precursores tipo hidrotalcitas
N. Pino1, G. Hincapié2, and D. López3
1 Chemical Engineer. Affiliation: Ph.D student, Universidad de Antioquia, Medellín, Colombia. E-mail: natalia.pino@udea.edu.co
2 Chemist, PhD. in Chemistry. Affiliation: Professor-Researcher, Politécnico Colombiano Jaime Isaza Cadavid, Medellín, Colombia. E-mail: gmarcela.hincapie@udea.edu.co
3 Chemist, PhD. in Chemistry. Affiliation: Professor-Researcher, Universidad de Antioquia, Medellín, Colombia. E-mail: diana.lopez@udea.edu.co
How to cite: Pino, N., Hincapié, G., and López, D. (2017). Hydrodeoxyge-nation of furfuryl alcohol over Cu/MgAl and Cu/ZnAl catalysts derived from hydrotalcite-like precursors. Ingeniería e Investigación, 37(1), 34-42. DOI: 10.15446/ing.investig.v37n1.57991.
Received: June 10th 2016 Accepted: February 1st 2017
ABSTRACT
The aqueous phase hydrodeoxygenation (HDO) of furfuryl alcohol over Cu/MgAl and Cu/ZnAl catalysts with different Mg/Al and Zn/ Al molar ratios, were investigated. Mg-Al and Zn-Al mixed oxides derived from hydrotalcites precursors were used as supports, which were impregnated with an aqueous solution of copper nitrate by incipient wetness impregnation. The HDO reaction was carried out in a typical batch reactor at 5 MPa of H2 and 200 °C for 4 h. Among the catalysts studied, the Cu/MgAl-0.5 catalyst exhibited the higher furfuryl alcohol conversion (86 %) and yield of cyclopentanol (35 %), which is the reaction product with the highest hydrogen-carbon (H/C) ratio. With the Cu/MgAl-3 catalyst a high cyclopentanone yield (67 %) was achieved. The results obtained, showed that copper supported on mixed oxides catalysts derived from hydrotalcite precursors are a promising alternative to improve the bio-oil quality.
Keywords: Hydrotalcites, cyclopentanol, furfuryl alcohol, copper, bio-oil upgrading.
RESUMEN
Se investigó la hidrodesoxigenación(HDO) del furfuril alcohol sobre catalizadores de Cu/MgAl y Cu/ZnAl con diferentes relaciones molares Mg/Al y Zn/Al. Óxidos mixtos de Mg-Al y Zn-Al derivados de precursores tipo hidrotalcitas se usaron como soporte, los cuales fueron impregnados con una solución acuosa de nitrato de cobre, mediante el método de impregnación húmeda. La reacción de hidrodesoxigenación (HDO) se realizó en un reactor tipo batch a 5 MPa de H2 y 200 °C por 4 h. Entre los catalizadores estudiados, el catalizador de Cu/MgAl-0.5 exhibió la más alta conversión del furfuril alcohol (86 %) y rendimiento hacia ciclopentanol (35 %), el cual es el producto de reacción con mayor relación hidrógeno-carbono (H/C). Con el catalizador de Cu/MgAl-3 se logró un alto rendimiento hacia ciclopentanona (67 %). Los resultados obtenidos, mostraron que los catalizadores de óxidos mixtos derivados de precursores tipo hidrotalcitas son una alternativa prometedora para mejorar las características del bio-oil.
Palabras clave: Hidrotalcitas, ciclopentanol, furfuril alcohol, cobre, mejora del bio-aceite.
Introduction
In recent years, effective chemical conversion of renewable biomass into fuels and chemicals has received significant attention worldwide. The use of lignocellulosic biomass is an attractive alternative to replace non-renewable fossil resources, given its low cost, abundance, sustainability and low environmental impact. New technologies to transform biomass into valuable chemicals and liquid transportation fuels are widely studied (Huber, Iborra, & Corma, 2006; Zacher, Olarte, Santosa, Elliott, & Jones, 2014).
The bio-oil obtained from biomass has high oxygen content in the form of short oxygenated compounds such as carboxylic acids, aldehydes and ketones which generate immiscibility, low heating value, corrosiveness and high viscosity. The high oxygen content of bio-oil, usually 20 to 50 wt%, changes its physical and chemical properties which directly affect its combustion behavior (Ruddy et al., 2014).
Catalytic hydrodeoxygenation (HDO) is proposed as an alternative to enhance bio-oil properties by removing oxygen and adding hydrogen to the oxygenated compounds. This catalytic bio-oil upgrading requires high hydrogen pressure (7-20 MPa) and temperature (160 °C-300 °C) to convert the low reactivity compounds in free oxygen products (Z. He & Wang, 2012). The main challenge of HDO processes is the development of a highly active and selective catalyst which can remove a high degree of oxygen with minimum hydrogen consumption (Resasco & Crossley, 2015).
The conventional HDS and HDN catalysts such as Co-Mo/ Al2O3 and NiMo/Al2O3 have been studied in the HDO of bio-oil with some drawbacks: they release sulfur oxides to the atmosphere, add soluble sulfur compounds to bio-oil and are deactivated due to coke formation and catalyst poisoning. Different catalysts have been evaluating for bio-oil upgrading, but they deactivated quickly. Due to the complexity and cost of the process to improve bio-oil properties, some strategies have been adopted. One of the strategies is to separate bio-oil into aqueous phase and organic phase. This allows a better treatment of each fraction to produce either fuels or chemicals (Pham, Shi, & Resasco, 2014; Valle, Remiro, Aramburu, Bilbao, & Gayubo, 2015).
The aqueous phase of bio-oil has higher oxygen content such as sugars, anhydrosugars, alcohols, acetic acid, hydroxyacetone, furfural (FFR), furfuryl alcohol (FFA) and small amounts of guaiacol. The short oxygenated compounds like furfural (FFR) and furfuryl alcohol (FFA) have high vapor pressures and need to be upgraded in order to improve bio-oil quality (Resasco & Crossley, 2015). Since bio-oil is mostly composed of short-chain oxygenated compounds, the increment of their hydrogen-carbon ratio (H/C) will be a remarkable contribution.
The reactions involved in the conversion of furfural and its derivatives, generate valuable compounds such as cyclopentanone (CPON) and cyclopentanol (CPOL). Thus, the development of an active catalyst for the HDO of furfural would enable the production of valuable compounds and the improvement of bio-oil properties.
In this work, with the aim to understand the steps and mechanism of furfural hydrodeoxygenation, furfuryl alcohol (FFA) as reaction intermediate is investigated. Hronec et al. (Hronec, Fulajtarová, & Liptaj, 2012a) found that Pt/C and Pd/C catalysts had good performances in the selective synthesis of CPON and CPOL from furfuryl alcohol in aqueous solution. The best yield (55,7 mol% of CPON and 13,6 mol% of CPOL) was obtained when Pt/C was employed as the catalyst. Until now, some precious metal catalysts (Pt/C, Pd/C, and Ru/C) and Ni-based catalysts have been demonstrated good activities in the conversion of furfural or furfuryl alcohol to CPON.
Although some work has been done on the synthesis of CPON and CPOL from furfural or furfuryl alcohol, it will be important the development of new catalytic systems, especially with low cost, high selectivity and stability.
Mixed oxides catalysts derived from hydrotalcites seems to be a promising alternative due to their interesting properties for HDO reactions: high surface area, modulation of their acid-base properties, moderate hydrothermal stability and low cost (Nishimura, Takagaki, & Ebitani, 2013; Vaccari, 1998). Ternary mixed oxides such as Cu-Mg-Al, Co-Zn-Al, have been studied in the FFA hydrodeoxygenation reaction. High conversions and good yields of value-added products are achieved with long reaction time. The high dispersion and low reducibility of the metal species is a clear disadvantage of these catalysts because their hydrogenation capacity and surface area decrease (Guo et al., 2014; Zhou, Zeng, Zhu, Xiao, & Xiao, 2014).
With the purpose to achieve a good hydrogenation function and take advantage of other properties of mixed oxides, in the present work, catalysts of copper supported on calcined hydrotalcites were prepared. Two types of layered double hydroxides, Mg-Al and Zn-Al, were evaluated in order to change the interaction of copper species with the cations of the support. The efficiency of the catalyst was studied in terms of both the conversion and the reaction yield.
Experimental
Materials
Cu(NO3)2-3H2O (99,5 %%, Merck), Mg(NO3)2-6H2O (98 %, Panreac), Zn(NO3)2-6H2O (98,0 %-102 %, Panreac), Al(NO3)3-9H2O (98,0 °%-102 %%, Panreac), Na2CO3 (99,9 %, Merck), NaOH (>99,0 %%, Merck), and furfuryl alcohol (98 %, Sigma-Aldrich). All chemicals were used as received without further purification.
Synthesis of catalyst
The Cu/MgAl mixed oxides were obtained through the impregnation of commercial hydrotalcites PURAL® MG70 and PURAL® MG30 supplied by SASOL, Germany at Mg/Al molar ratios of 3,0 and 0,5, respectively. Before calcination, the commercial hydrotalcites were impregnated with an aqueous solution of copper nitrate by incipient wetness impregnation. The nominal copper supported was 20 wt%. The resulting materials were calcined at 400 °C for 4 h and then reduced in H2 atmosphere at 400 °C for 4 h.
In order to synthesize the Cu/ZnAl mixed oxides, hydrotalcites with different Zn/Al molar ratios (Zn/Al = 0,5 and 3,0) were prepared by co-precipitation method (J. He, Wei, Li, & Kang, 2006). An aqueous solution of Zn(NO3)2.6H2O and Al(NO3)3.9H2O which contains the desired Zn/Al molar ratio, and a second solution of NaOH (2 M) were simultaneously added drop-wise to a solution of Na2CO3 (2 M). The pH reaction was maintained at 9,910,1. During the precipitation, the reaction solution was kept at 65 °C with vigorous stirring for 4 h, and then aged without stirring at 65 °C for 24 h. Finally, the resulting slurry was filtered, washed several times with deionized water to remove salts of sodium nitrate and dried at 110 °C overnight (Corma, Fornés, & Rey, 1994; Cosimo, Diez, & Xu, 1998; Shen, Kobe, Chen, & Dumesic, 1994).
The obtained solids were also impregnated by incipient wetness impregnation with Cu(NO3)2-3H2O in order to obtain 20 wt% of copper supported. The materials were calcined at 400 °C for 4 h in a static air atmosphere and then reduced in a H2 atmosphere at 400 °C for 4 h before reaction. The reduced catalysts are labeled as Cu/MgAl-x and Cu/ZnAl-x, where x represented the MII/ MIII molar ratios of the catalyst's support.
Characterization of catalyst
The Zn-Al hydrotalcites were characterized through X-ray diffraction (XRD) to ensure that are layered structures. X-ray diffraction studies were carried in an Empyrean (PANalytical) diffractometer using Cu Ka radiation (λ= 1,5406 Å). The data were recorded over 2θ ranges of 5-90° with a step size of 0,039°. Diffraction peaks of crystalline phases were compared with those of standard compounds reported in the JCPDS Data File.
The morphologies of the mixed oxides were examined through Scanning Electron Microscopy (SEM) by means of the instrument (JSM-6490LV) operated at 20 kV and with a depth of 10 microns. The Mg/Al and Zn/Al molar ratios were estimated as the average of the different values obtained by Energy-dispersive X-ray spectroscopy (EDX). EDX and mapping analysis were performed at randomly selected areas on the solid surfaces. The chemical composition of the mixed oxides derived from hydrotalcites was estimated using a Thermo Scientific ARL OPTIM'X wavelength dispersive X-ray fluorescence (XRF) spectrometer.
Hydrogen temperature-programmed reduction (H2-TPR) studies were carried out using a Micromeritics Autochem II 2920 instrument. The calcined samples were placed in a U-shaped quartz reactor and pretreated in helium at 400 °C before reduction in order to remove humidity and clean the surface. After cooling down to room temperature and introducing the reduction mixture of 10 % H2/Ar, the sample was heated at a rate of 10 °C min-1 from room temperature to 900 °C. The hydrogen consumption was estimated from the area under the peak and taking in count the calibration of TCD signal.
Finally, BET surface areas of the solids were measured by nitrogen adsorption using ASAP 2020 Micromeritics® apparatus. Before each measurement the samples were dehydrated in vacuum at 400 °C for 1 h.
Hydrodeoxygenation of furfuryl alcohol
Hydrodeoxygenation of furfuryl alcohol (FFA) was carried out in a 30 mL stainless autoclave equipped with a mechanical stirrer. For the heterogeneous catalytic reaction, 15 mL of water, 1 mL of furfuryl alcohol and 0,2 g of catalysts were added into the reactor vessel. The experiments were performed at 5 MPa and 200 °C. Reaction time was 4 h with a stirring speed of 600 rpm.
Finally, the reaction was quenched in a water bath. The reaction products were separated from the catalyst by filtration. Identification of products in the collected liquid was performed with a gas chromatographer (GC-MS QP2010 Plus Shimadzu) equipped with a DB-5 capillary column (30 m x 0,25 mm x 0,10 μ.m).
The separated catalysts were dried without additional treatments (avoiding the reconstruction of the mixed oxides) in order to be reused in new hydrodeoxygenation reactions. The mass balance of the reaction was done using the compounds identified by the GC-MS in the liquid phase.
Results and discussion
Characterization of catalyst
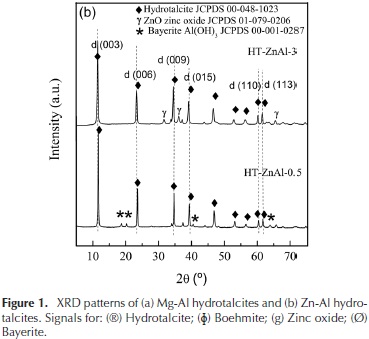
The XRD patterns of the Mg-Al and Zn-Al hydrotalcites are shown in Figure 1.
The peaks assigned to (003), (006), (009), (110) and (113) planes are characteristic of hydrotalcite materials having a layered structure (Sharma & Kushwaha, 2007). Small amount of boehmite in the HT-Mg-Al-0.5 was detected which cause a decrease in the material crystallinity. Also, weak peaks of ZnO and bayerite were identified in the HT-Zn-Al samples (Thevenot, Szymanski, & Chaumette, 1989).
The reflections assigned to (003) and (006) planes could be used to calculate the basal spacing between the layers d. The reflections assigned to (110) plane could be used to calculate the unit cell dimension a, (where a = 2d(110)). The crystallite size of hydrotalcite was calculated using Scherrer's equation (F. Cavani, F. Trifiro, 1991). Silicon was used as a standard sample. The calculation results are shown in Table 1.
As shown in Table 1, the parameter a, which is a function of the cation-cation distance in the hydroxide layers, was in the range 3,03-3,07 Å. This parameter decreases as Al+3 content increases due to the progressive isomorphic replacement of Mg2+ (ionic radii of 0,65 Å) by Al3+ (ionic radii of 0,50 Å) which causes a contraction in the structure (Cosimo et al., 1998; Shannon, 1976).
Also, it is observed that the interlaminar space d(003) increases with increasing Mg+2 or Zn+2 hydrotalcites content, this is associated with a reduction of the columbic interaction between the positive brucite layers and the interlaminar anions (Cantrell, Gillie, Lee, & Wilson, 2005).
The crystallite sizes of the hydrotalcites changed with the Mg/Al and Zn/Al molar ratios because it led to a modification in the interatomic distances, bond lengths and angles of the structures (Miyata, 1980; Pu & Zhang, 2005; Shannon, 1976; Thevenot et al., 1989).
It can be observed that the crystallinity of the samples increases with the Mg and Zn content. This is because no segregated phases of boehmite and bayerite were detected in these solids.
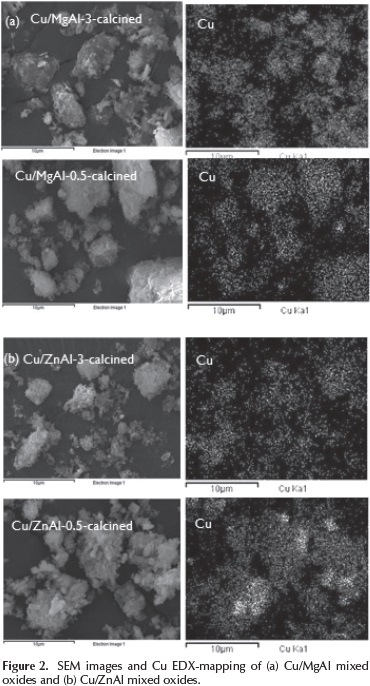
The morphologies and elemental mapping of the calcined catalysts are showed in Figure 2.
Agglomerates particles with undefined form were obtained due to the partial collapse of the layered structure of the hydrotalcites.
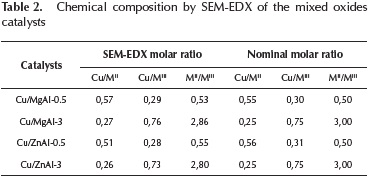
The elemental composition of the different catalytic systems determined by SEM-EDX is shown in Table 2.
The chemical compositions of the mixed oxides reported in Table 2 show that the experimental data are consistent with the nominal values. The values of Mg/Al and Zn/Al molar ratios determined by EDX were closely to the nominal values.
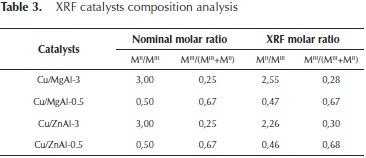
With the purpose to validate the compositional results obtained by EDX, a XRF analysis was done for the mixed oxides catalysts. These results are shown in Table 3.
The composition values obtained by XRF are also similar to the nominal composition of the synthetized materials.
The catalysts showed a uniform copper distribution on the surface. Mapping images describes some areas with higher density of copper particles associated with the impregnation method used (incipient wetness impregnation).
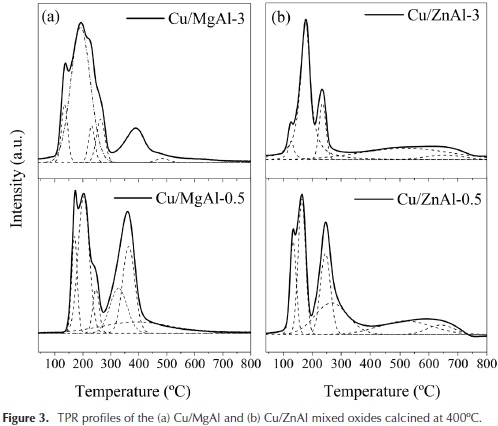
The reducibility of the catalyst calcined at 400 °C was investigated by TPR measurements, and the obtained profiles are shown in Figure 3.
As shown in Figure 3, the Cu/MgAl oxides broad peaks were deconvoluted into two distinct regions, with the corresponding temperature range of 100 - 300 and 300 - 450 °C. In the low-temperature region, the peaks can be attributed to the reduction of the highly reducible copper from well-dispersed CuO. The more intense peaks at higher temperature of 360 °C are attributed to the reduction of copper in CuO particles interacting more strongly with the support (Jiang et al., 2012; Kovanda, Jirátová, Rymes, & Kolousek, 2001; Tanasoi et al., 2009; Villaverde, Garetto, & Marchi, 2015). The same behavior could be ascribed to the Cu/ZnAl oxides.
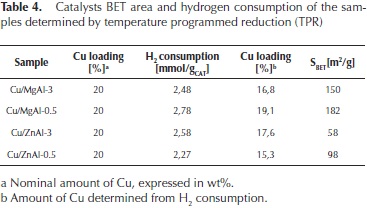
It is interesting to note that the Cu/MgAl oxides required higher reducibility temperatures than the Cu/ZnAl oxides. This behaviour depends of the interaction of copper species with the supports. For the Cu/ZnAl oxide, the deconvoluted TPR peaks observed at higher temperatures (450-700 °C) correspond to residual carbonates decomposition (Barrault et al., 2004). The corresponding H2 consumptions and the catalysts BET areas are reported in Table 4.
From these values, and assuming a stoichiometry of H2/ Cu = 1, the nominal amount of Cu2+ ions reduced was determined. These results confirm that, in all of the cases, most of the Cu2+ ions can be totally reduced to Cu0.
Hydrodeoxygenation of furfuryl alcohol
The FFA hydrodeoxygenation at 200 °C and 5 MPa was evaluated on reduced Cu/MgAl and Cu/ZnAl catalysts.
High hydrogen pressure is used in order to achieve better hydrogen solubility. It is important to note that hydrogen solubility in water (simulating bio-oil conditions) is too low compared to the solubility in the conventional hydrotreating of fuels (Hrnčič, Venderbosch, Škerget, Ilić, & Knez, 2013).
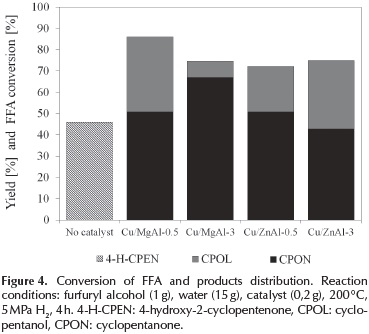
The conversion and product distribution of the furfuryl alcohol hydrodeoxygenation over copper supported on mixed oxides catalysts are shown in Figure 4.
As shown in Figure 4, copper-based catalysts presented a good activity in the aqueous phase hydrodeoxygenation of furfuryl alcohol. The main products detected from the reaction were cyclopentanone (CPON), cyclopentanol (CPOL) and 4-hydroxy-2-cyclopentenone (4-H-CPEN).
The main product of the furfuryl alcohol conversion in water without catalyst was 4-hydroxy-2-cyclopentenone (4-H-CPEN). This result is in agreement with previous works (Hronec, Fulajtárova, & Soták, 2014) which report the spontaneously furfuryl alcohol conversion to 4-HCP in aqueous medium at temperatures above 110 °C, in the absence of H2 pressure or catalyst.
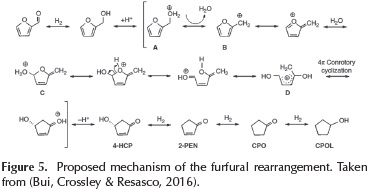
The formation of 4-H-CPEN without catalyst is related to the effect of water, which plays a key role in the furfuryl alcohol ring rearrangement. This reaction has initially been reported by Piancatelli et al.(Piutti & Quartieri, 2013) upon observing the ring rearrangement of 2-furylcarbinol in an acidic aqueous system and has subsequently been referred as a Piancatelli rearrangement. Basically, the ring rearrangement step does not need catalyst, just water and proper temperature to promote the water self-dissociation. The dissociation of the water generates the formation of the hydronium ions (H3O+) which are responsible to catalyze the furan ring rearrangement.
The reaction pathway for the ring rearrangement includes: the protonation of the OH group on furfuryl alcohol to form a carbocation A (Figure 5). The unstable nature of the protonated furfuryl alcohol in water promotes its decomposition into B. Then, the nucleophilic attack of water onto the ring form the intermediate C, as is shown in Figure 5. Consecutively, C undergoes ring opening to generate species D, which can undergo 4π-conrotatory cyclization to facilitate the ring closure. Then, the successive deprotonation of this species yields 4-hydroxy-2-cyclopentenone (4-H-CPEN). Finally, in the presence of the catalyst and H2, this intermediate 4-HCPEN, could undergo hydrogenation to form CPON and sequential CPOL.
As is reported in previous works (Hronec, Fulajtárova, et al., 2014) and(Hronec, Fulajtarová, & Liptaj, 2012b), when the reaction is conducted at high H2 pressures over metal catalysts, the formation of hydrogenated byproducts such as THFA (tetrahydrofurfuryl alcohol) and MTHF (2-methyltetrahydrofuran) is improved. This suggests that under these conditions, the furan ring hydrogenation starts to compete with the furfuryl alcohol rearrangement, therefore the selectivity to ring rearrangement products is diminished.
With the Cu/MgAl-0.5 catalyst, the yield of CPOL was 35 % and CPON 51 %; and with the Cu/MgAl-3, the yield of CPOL was 7,7 % and CPON 67 %. Thus, the Cu/MgAl-0.5 catalyst achieved a higher hydrodeoxygenation of the furfuryl alcohol, which could be attributed to its high hydrogen consumption. Contrarily, with the Cu/ZnAl catalysts, the higher yield of cyclopentanol was achieved with the Cu/ ZnAl-3 material (32 % of CPOL) than with the Cu/ZnAl-0.5 catalyst (21 % of CPOL). A high yield of CPOL are associated with both: the amount of reducible copper species and the acidity of the supports(Kovanda et al., 2001).
It can be observed in Figure 4., that furfuryl conversion was higher with the Cu/MgAl catalysts than with Cu/ZnAl. The low surface area and the low H2 consumption with the Cu/ZnAl catalysts are possible reasons to explain its lower activity compared with Cu/MgAl catalysts.
Correlating the catalysts hydrogen consumption and the yield of cyclopentanol, the Cu/MgAl-0.5 and Cu/ZnAl-3 catalysts exhibited the highest hydrogen consumption and yield of cyclopentanol. As it was mentioned, these results are correlated with the Cu0 species on the catalyst which are considered as the active component for the hydrogenation.
In addition, the chemical nature of the cations in the support and their interaction with the copper species affect the conversion and yield of the hydrodeoxygenation reaction products (Kovanda et al., 2001).
Based on previous studies (Xiong, Pham, & Datye, 2014) (Z. He & Wang, 2013) and the results discussed above, it is possible to argue that moderate acidity in the support enhances its hydrothermal stability, increases the rate of dehydration and hydrogenation reactions, decreases the polymerization of furfuryl alcohol and improves other catalyst properties (textural properties and metal dispersion). This explains the high yield of CPOL achieved over the Cu/MgAl-0.5 catalyst. By contrast, it is expected that high density of basic sites, decreases the catalyst hydrothermal stability and the hydrogenation activity.
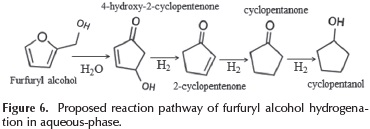
According to the products distribution showed in Figure 4, a scheme of the furfuryl alcohol conversion is proposed in Figure 6. This scheme is in agreement with the reaction pathway proposed by (Guo et al., 2014).
The first step is the interaction of furfuryl alcohol with water which causes a rearrangement in the furan ring to produce 4-hydroxy-2-cyclopentenone. Subsequently, these intermediates are hydrogenated to form cyclopentanone and cyclopentanol. The quantity of Cu0 species present and their interaction with the support promote the furfuryl alcohol hydrodeoxygenation reaction which is in agreement with our results.
Catalyst recyclability
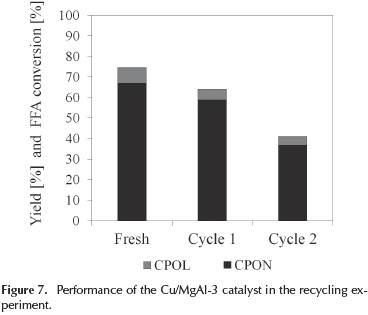
The stability of the Cu/MgAl-3 catalyst was examined. A batch of Cu/MgAl-3 was recycled twice and tested on the conversion of furfuryl alcohol. The results are illustrated in Figure 7.
As shown in Figure 7, the conversion of furfuryl alcohol decreases from 75 % to 41 % after two cycles. The yield of cyclopentanone and cyclopentanol decreased to 37 % and 4 % respectively. These results could be attributed to a progressively deactivation of the catalysts due to their capacity to recover a layered structure at 400 °C of calcination temperature (Hronec, Fulajtárová, & Soták, 2014). Previous studies have mentioned that mixed oxides derived from hydrotalcite type precursors are unstable until a calcination temperature of 500 °C and become more stable with increasing calcination temperature.
One of the most interesting and useful properties of hydrotalcites is their regeneration ability often called "memory effect". In the case of HDO reaction, a hydrothermal stable catalyst is required. The mixed oxide reconstruction decreases the catalyst surface area, the metal dispersion and catalyst activity. In order to improve the catalysts hydrothermal stability and also their recyclability, an increase in the calcination temperature will be necessary (Hronec, Fulajtárová, et al., 2014; Kovanda et al., 2001).
The recyclability of the Cu/ZnAl catalysts was very difficult due to agglomeration which could also contribute to their easy deactivation.
Mass Balance
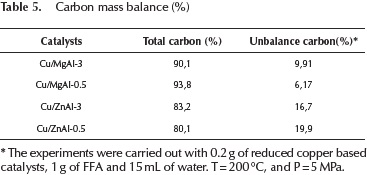
The carbon mass balance of the reaction was estimated using the products that were identified by GC-MS and the amount of furfuryl that was fed. The carbon balance was calculated as moles of carbon in products divided by the initial moles of carbon in the feeding (furfuryl alcohol). The values obtained are presented in Table 5.
The mass balance listed in Table 5 was reasonably well closed. The unbalance carbon is associated with furfuryl alcohol polymerization products that were not included in the balance. Difurfuryl ether was identified as the main product of the furfuryl alcohol polymerization.
Conclusions
Cu/MgAl and Cu/ZnAl catalysts exhibited high catalytic activity for the hydrodeoxygenation of furfuryl alcohol towards valuable compounds such as cyclopentanone and cyclopentanol.
Cu/MgAl-0.5 and Cu/ZnAl-3 catalysts presented the highest hydrogen consumption and yield of cyclopentanol, which could be attributed to more species of Cu0 available to hydrogenate. It is believed that the interaction of copper with the support affects the amount of copper reducible species (Cu+2) which in turn affects the pathways of the furfuryl alcohol hydrodeoxygenation. The amount of reducible copper species and their interaction with the support have great effect on the furfuryl alcohol conversion.
Acknowledgments
The authors acknowledge (i) Universidad de Antioquia for the financial support through Programa Sostenibilidad, (ii) Colciencias for financing the project 1115-569-33557. Natalia Pino acknowledges Universidad de Antioquia for her Ph.D. scholarship.
References
Barrault, J., Derouault, A., Courtois, G., Maissant, J., Dupin, J., Guimon, C., Dumitriu, E. (2004). On the catalytic properties of mixed oxides obtained from the Cu-Mg-Al LDH precursors in the process of hydrogenation of the cinna-maldehyde. Applied Catalysis A: General, 262(1), 43-51. [ Links ]
Bui, T. V, Crossley, S., & Resasco, D. E. (2016). Chemicals and Fuels from Bio-Based Building Blocks. ( and A. G. Fabrizio Cavani, Stefania Albonetti, Francesco Basile, Ed.) (First edit). [ Links ]
Cantrell, D. G., Gillie, L. J., Lee, A. F., & Wilson, K. (2005). Structure-reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis. Applied Catalysis A: General, 287(2), 183-190. [ Links ]
Corma, A., Fornés, V., & Rey, F. (1994). Hydrotalcites as base catalysts: influence of the chemical composition and synthesis conditions on the dehydrogenation of isopropanol. Journal of Catalysis, 148, 205-212. [ Links ]
Cosimo, J. Di, Diez, V., & Xu, M. (1998). Structure and surface and catalytic properties of Mg-Al basic oxides. Journal of Catalysis, 510, 499-510. [ Links ]
F. Cavani, F. Trifiro, A. V. (1991). Hydrotalcite-type anionic clays: Preparation, properties and applications. Catalysis Today, 11, 173-301. [ Links ]
Guo, J., Xu, G., Han, Z., Zhang, Y., Fu, Y., & Guo, Q. (2014). Selective Conversion of Furfural to Cyclopentanone with CuZnAl Catalysts. ACS Sustainable Chemistry & Engineering, 2(10), 2259-2266. [ Links ]
He, J., Wei, M., Li, B., & Kang, Y. (2006). Preparation of layered double hydroxides. Layered Double Hydroxides, 89-119. [ Links ]
He, Z., & Wang, X. (2012). Hydrodeoxygenation of model compounds and catalytic systems for pyrolysis bio-oils upgrading. Catalysis for Sustainable Energy, 1, 28-52. [ Links ]
He, Z., & Wang, X. (2013). Required catalytic properties for alkane production from carboxylic acids: Hydrodeoxyge-nation of acetic acid. Journal of Energy Chemistry, 22(6), 883-894. [ Links ]
Hrnčič, M. K., Venderbosch, R. H., Škerget, M., Ilić, L., & Knez, Z. (2013). Phase equilibrium data of hydrogen in pyrolysis oil and hydrogenated pyrolysis oil at elevated pressures. The Journal of Supercritical Fluids, 80, 86-89.
Hronec, M., Fulajtarová, K., & Liptaj, T. (2012a). Effect of catalyst and solvent on the furan ring rearrangement to cy-clopentanone. Applied Catalysis A: General, 437-438, 104-111. [ Links ]
Hronec, M., Fulajtarová, K., & Liptaj, T. (2012b). Effect of catalyst and solvent on the furan ring rearrangement to cy-clopentanone. Applied Catalysis A: General, 437-438, 104-111. [ Links ]
Hronec, M., Fulajtárova, K., & Soták, T. (2014). Highly selective rearrangement of furfuryl alcohol to cyclopentanone. Applied Catalysis B: Environmental, 155, 294-300. [ Links ]
Hronec, M., Fulajtárová, K., & Soták, T. (2014). Kinetics of high temperature conversion of furfuryl alcohol in water. Journal of Industrial and Engineering Chemistry, 20(2), 650-655. [ Links ]
Huber, G., Iborra, S., & Corma, A. (2006). Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chemical Reviews, 106(9), 4044-98. [ Links ]
Jiang, Z., Kong, L., Chu, Z., France, L. J., Xiao, T., & Edwards, P. P. (2012). Catalytic combustion of propane over mixed oxides derived from CuxMg3-xAl hydrotalcites. Fuel, 96(x), 257-263. [ Links ]
Kovanda, F., Jirátová, K., Rymes, J., & Kolousek, D. (2001). Characterization of activated Cu/Mg/Al hydrotalcites and their catalytic activity in toluene combustion. Applied Clay Science, 18, 71-80. [ Links ]
Miyata, S. (1980). Physico-Chemical Properties of Synthetic Hydrotalcites in Relation to Composition. Clays and Clay Minerals, 28(1), 50-56. [ Links ]
Nishimura, S., Takagaki, A., & Ebitani, K. (2013). Characterization, synthesis and catalysis of hydrotalcite-related materials for highly efficient materials transformations. Green Chemistry, 15(8), 20-26. [ Links ]
Pham, T. N., Shi, D., & Resasco, D. E. (2014). Evaluating strategies for catalytic upgrading of pyrolysis oil in liquid phase. Applied Catalysis B: Environmental, 145, 10-23. [ Links ]
Piutti, C., & Quartieri, F. (2013). The piancatelli rearrangement: New applications for an intriguing reaction. Molecules, 18(10), 12290-12312. [ Links ]
Pu, M., & Zhang, B.-F. (2005). Theoretical study on the micros-tructures of hydrotalcite lamellae with Mg/Al ratio of two. Materials Letters, 59(27), 3343-3347. [ Links ]
Resasco, D. E., & Crossley, S. P. (2015). Implementation of concepts derived from model compound studies in the separation and conversion of bio-oil to fuel. Catalysis Today, 257, 185-199. [ Links ]
Ruddy, D. a., Schaidle, J. a., Ferrell III, J. R., Wang, J., Moens, L., & Hensley, J. E. (2014). Recent advances in heterogeneous catalysts for bio-oil upgrading via "ex situ catalytic fast pyrolysis": catalyst development through the study of model compounds. Green Chem., 16(2), 454-490. [ Links ]
Shannon, R. (1976). Revised effective ionic radii and systematic studies of interatomic distances in halides and chalco-genides. Acta Crystallographica Section A: Crystal Physics, Diffraction, Theoretical and General Crystallography, 32, 751-767. [ Links ]
Sharma, S., & Kushwaha, P. (2007). Effect of hydrothermal conditions on structural and textural properties of synthetic hydrotalcites of varying Mg/Al ratio. Industrial & Engineering Chemistry Research, 46(14), 4856-4865. [ Links ]
Shen, J., Kobe, J., Chen, Y., & Dumesic, J. (1994). Synthesis and surface acid/base properties of magnesium-aluminum mixed oxides obtained from hydrotalcites. Langmuir, (14), 3902-3908. [ Links ]
Tanasoi, S., Tanchoux, N., Urdã, A., Tichit, D., Sãndulescu, I., Fajula, F., & Marcu, I.-C. (2009). New Cu-based mixed oxides obtained from LDH precursors, catalysts for methane total oxidation. Applied Catalysis A: General, 363(1-2), 135-142. [ Links ]
Thevenot, F., Szymanski, R., & Chaumette, P. (1989). Preparation and characterization of Al-richH Zn-Al hydrotalcite compounds. Clays and Clay Minerals, 37(5), 396-402. [ Links ]
Vaccari, A. (1998). Preparation and catalytic properties of cationic and anionic clays. Catalysis Today, 41(1-3), 53-71. [ Links ]
Valle, B., Remiro, A., Aramburu, B., Bilbao, J., & Gayubo, A. G. (2015). Strategies for maximizing the bio-oil valorization by catalytic transformation. Journal of Cleaner Production, 88, 345-348. [ Links ]
Villaverde, M. M., Garetto, T. F., & Marchi, A. J. (2015). Liquid-phase transfer hydrogenation of furfural to furfuryl alcohol on Cu-Mg-Al catalysts. Catalysis Communications, 58, 6-10. [ Links ]
Xiong, H., Pham, H. N., & Datye, A. K. (2014). Hydrothermally stable heterogeneous catalysts for conversion of biore-newables. Green Chem., 16(11), 4627-4643. [ Links ]
Zacher, A. H., Olarte, M. V., Santosa, D. M., Elliott, D. C., & Jones, S. B. (2014). A review and perspective of recent bio-oil hydrotreating research. Green Chem., 16(2), 491-515. [ Links ]
Zhou, M., Zeng, Z., Zhu, H., Xiao, G., & Xiao, R. (2014). Aqueous-phase catalytic hydrogenation of furfural to cyclopentanol over Cu-Mg-Al hydrotalcites derived catalysts: Model reaction for upgrading of bio-oil. Journal of Energy Chemistry, 23(1), 91-96. [ Links ]