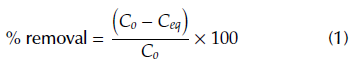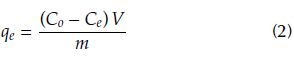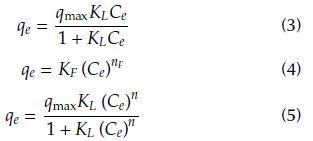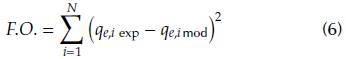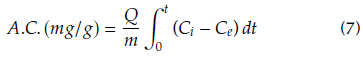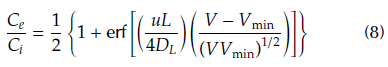Introduction
In recent years, a lot of waste is generated from industrial and human daily activities, producing toxic compounds that pollute the environment and cannot be degraded by nature. These residues include the so-called emerging contaminants (EC), which consistin a wide variety of chemical compounds, such as pharmaceutical drugs, personal care products, surfactants, plasticizers and chemical additives from industrial processes, that are not considered in the current monitoring programs for wastewater treatment (Jiang, Xiao, Wang, Wang, and Cai, 2015). Pharmacological residues represent the highest percentage of EC, which is a big concern for public health, since the effect of chronic exposure is unknown. (Tejada, Quiñonez, and Peña, 2014) One of the most commonly used drugs is the acetylsalicylic acid (ASA), which serves as anti-inflammatory, analgesic, antipyretic and antiplatelet agent (Mukherjee, Ray, and Barghi, 2016). There are various methods for the removal of this type of contaminant from wastewater (Roig Bondia, 2013), but adsorption on carbonaceous materials appears to be the best and the most frequent strategy used, since it is efficient, economic, and environment-friendly (Vargas, 2013). Among these materials, there is a group called carbon xerogels, which have a mesoporous structure and are able to adsorb larger molecules, such as emerging contaminants or dyes (/Álvarez, Ribeiro, Gomes, Sotelo, and Garcia, 2015). In the literature, some studies have focused on the use of carbon xerogels as adsorbent materials. For example, Álvarez et al. (2015) studied the elimination of caffeine and diclofenac using carbon xerogels. The maximum adsorption capacity was 182,5 mg/g for caffeine and 80,0 mg/g for diclofenac. Carabineiro, Thavorn-amornsri, Pereira, Serp and Figueiredo (2012) used activated carbon, carbon nanotubes and carbon xerogel for the adsorption of ciprofloxacin, obtaining adsorption capacities from 112 to 135 mg/g. The carbon nanotube sample was the material with the highest adsorption capacity per unit area, which was attributed to its high surface area and electron-donor capacity.
The modification of the adsorbent material with TiO2 nanoparticles offers additional advantages. Some of them are a synergistic effect with the adsorbent compound, through the increase of surface area as a function of the reduced particle size; the development of mesopores; the exposure of a more active crystalline phase; and the possibility of the subsequent photodegradation of the pollutant, avoiding the generation of residual products (Bailon-Garcla et al., 2017; Lopez-Muñoz, Arencibia, Cerro, Pascual, and Melgar, 2016). Authors such as Borges, Garcia, Hernández, Ruiz-Morales and Esparza, (2015) studied the removal of paracetamol using a photoreactor, where TiO2 was supported in glass spheres. The photocatalytic activity reached a high photodegradation between 99% and 100% after 4 hours of irradiation. Additionally, Bailón-García et al. (2017) studied a series of carbon xerogels-TiO2 composites that were used as photocatalysts and adsorbents of dyes. They concluded that the adsorption of the dye is controlled by the mesopore volume, which augmented with the increasing percentage of titanium oxide present in the carbonaceous composite.
In the last two decades, related studies on the implementation of microdevices have shown that they have several advantages on conventional processes. Their small size and large surface to volume ratios leads to a lower driving force required for mass and heat transfer, thus smaller unit volumes are necessary (Kenig, Su, Lautenschleger, Chasanis, and Gmnewald, 2013). Few studies about adsorption in microchannels have been reported. For instance, El-Qada, Abdelghany and Magdy (2013) studied the adsorption of dyes in a fixed-bed microcolumn using activated carbon. Their results show the effect of initial dye concentration, column diameter and particle size on the microcolumn performance. Sharmaand Tiwari (2016) studied the removal of Cu2+ using acrylamide-co-maleic acid in a fixed-bed microcolumn. From these experiments, they observed that an increase in the flow rate and inlet concentration of the metal also increased the adsorption capacity up to a 98 % of Cu2+, with the possibility of bed regeneration for a new application.
In this work, we report the removal of acetylsalicylic acid using activated carbon xerogels modified with TiO2 nanoparticles. Batch adsorption experiments were performed to obtain the equilibrium curves and determine the effect of the adsorbent dose, pH of the solution, and initial concentration of the adsorbate. Additionally, breakthrough curves were obtained for continuous removal using packed-bed microcolumns at different operating conditions.
Experimental
Reagents
Acetyl salicylic acid (ASA) was purchased from Sigma-Aldrich in analytical grade (> 99%). Formaldehyde (37% in water, stabilized with 10% methanol), resorcinol (≥ 99%) and sodium hydroxide (98 %) were purchased from Merck. Titanium (IV) isopropoxide (95 %) and tetraethyl orthosilicate (98 %) were acquired from AlfaAesar, while dimethylsulfoxide (99%) was obtained from Panreac.
Synthesis of carbon xerogel
Following the experimental procedure proposed by Álvarez et al. (2015), carbon xerogel (CX) was obtained by polycondensation of resorcinol with formaldehyde (molar ratio 1:2). In atypical experiment, 9,91 g of resorcinol was dissolved in distilled water, with the addition of 13,5 mL of formaldehyde solution. In order to achieve the desired initial pH of the precursor solution (pH = 6,1), sodium hydroxide solution was added drop wise under continuous stirring and pH monitoring. Later, the resulting solution was taken to an oven at 85 °C for 3 days. The obtained gel was then dried in an oven for several days at 60 °C, with a pressure ranging between 103 Pa and 105 Pa. Finally, the gel was calcined in the presence of N2 at 900 °C for one hour and activated with CO2 at 840 °C for 2 hours.
Synthesis of nanoparticles and modification of the xerogel
The synthesis of titanium dioxide (TiO2) nanoparticles was performed using the green chemistry method, where a chemical reduction of titanium tetraisopropoxide was made using an aqueous extract obtained from leaves of lemongrass. For this synthesis, 850 mL of lemongrass extract were mixed with 1,33 mL of titanium tetraisopropoxide, shaking at 175 rpm for 24 hours. Afterward, nanoparticles were precipitated by centrifugation at 5000 rpm and dried at room temperature. To obtain the desired crystal structure, the TiO2 nanoparticles were calcined at 450 °C for 3 hours. To modify the xerogel with the nanoparticles, 1,0 g of the carbonaceous material was initially prepared and suspended in 20 mL of organic solvent dimethylsulfoxide (DMSO), which was kept under slow shaking for 24 h at 30 °C. Then, 3 mL of tetraethylorthosilicate (TEOS) were added to the mixture and left for 48 h at room temperature. Subsequently, 0,5 g of the synthesized nanoparticles were added to the suspension, and the mixture was placed on an orbital shaker for 12 hours at 30 °C. Afterward, the modified material was precipitated using a centrifuge at 5000 rpm for 15 min and then washed with ethanol (Bitar Castro and Mejía Meza, 2015).
Characterization of nanoparticles and carbon xerogels
The physicochemical, morphological and surface properties of the carbonaceous materials and TiO2 nanoparticles were characterized by Scanning Electron Microscopy (SEM) in a JCM-6000 Plus microscope (JEOL Ltd, Japan). BET surface area measurements were taken in an ASAP2020 Plus System (Micromeritics, USA) and X-Ray Diffraction (XRD) was performed using Empyrean Series II X-ray diffractometer (Malvern Panalytical, USA). Finally, Fourier transform infrared spectroscopy was made in a Shimadzu Model IRAffinity-IS FTIR.
Dose curve
Carbon xerogels quantities between 25 and 250 mg were taken and put in contact with 25 mL of ASA aqueous solution, with concentration of 100 mg/L. The solution pH was not adjusted. The suspensions were continuously agitated at room temperature, at 150 rpm, for 7 h until equilibrium was reached. Then, suspensions were filtered, and the ASA equilibrium concentrations were determined at λ = 280 nm, using a UV-1800 spectrophotometer (Shimadzu, Japan). The removal efficiency at equilibrium was calculated using Equation (1).
where C o (mg/L) is the liquid-phase concentration of ASA at t = 0 and C eq is the equilibrium concentration.
Effect of solution pH
The pH of the experimental solutions was adjusted from 2 to 11 by addition of HCl and NaOH solutions. The ASA concentration was fixed at 100 mg/L. Tests were carried out at room temperature and agitated at 150 rpm with a contact time of 7 h. Once this time was elapsed, suspensions were filtered, and the ASA concentrations were determined at λ = 280 nm, using a UV-1800 spectrophotometer (Shimadzu, Japan).
Adsorption isotherms
Batch adsorption studies allow to obtain the equilibrium data for a fixed temperature of the mixture. In this test, different ASA solutions were prepared at concentrations between 10 and 200 mg/L, at room temperature. The doses of adsorbent and pH of the solution were taken from the previous results. After a contact time equivalent to 2 h, the solutions were filtered and analyzed in a UV-1800 spectrophotometer (Shimadzu, Japan) at a wavelength of 230 nm. This determined the adsorption of ASA on the evaluated material (Gómez Rengifo, 2013). Mass of ASA adsorbed per mass of each utilized material qe , calculated according to Equation (2), is presented as a function of the ASA concentration Ce.
where Co and Ce (mg/L) represent the initial and the equilibrium concentration of the adsorbate in the solution, respectively. V(L) is the volume of the solution, and m(g) is the dry mass of the adsorbent.
Langmuir (Eq. 3), Freundlich (Eq. 4) and Langmuir-Freundlich (L-F) (Eq. 5) adsorption models were used to fit the experimental equilibrium adsorption data (Álvarez et al., 2015)
where C e (mg/L) represents the equilibrium concentration in the aqueous phase, q e (mg/g) the equilibrium adsorption capacity and q max (mg/g) the maximum adsorption capacity according to Langmuir and Langmuir-Freundlich models. KL (L/mg) is a parameter related to the adsorption intensity for Langmuir and Langmuir-Freundlich equations and n is a constant associated with the heterogeneity of the adsorber in the Langmuir-Freundlich model. KF (L/g) and n F are parameters that define the adsorption capacity and adsorption intensity in the Freundlich model. The parameters of the models can be estimated by nonlinear regression analysis, minimizing the following objective function (Eq. 6):
Packed-bed microcolumn experiments
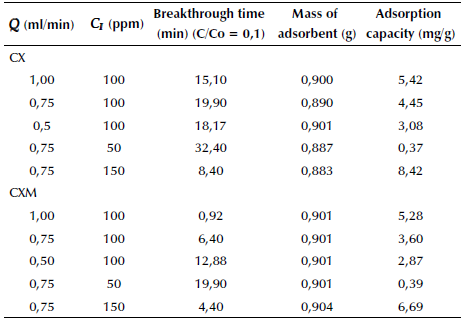
A capillary glass tube of 1,0 mm internal diameter and 10 cm length was used as adsorption microcolumn. The adsorbent material was ground and sieved to obtain an average particle diameter of 300 pm, which was packed in the column with a bed length of 5 cm. Solutions of different concentrations of ASA (50,100 and 150 mg/L) were continuously fed into the microcolumn at different flow rates (0,5, 0,75 and 1,0 mL/min) using a NE-1000 syringe pump (New Era Pumps, USA). The change in time of the ASA concentration in the column effluent was determined by continuous UV measurements at a wavelength λ = 230 nm in a UV-1800 spectrophotometer (Shimadzu, Japan). The column was kept at room temperature. The microcolumn performance was evaluated through the breakthrough curves of the continuous fixed bed system and the adsorption capacity of the adsorbent. The latter is defined as the ratio of the total adsorbed contaminant in the bed to the total amount of the adsorbent material packed in the column (Eq. 7).
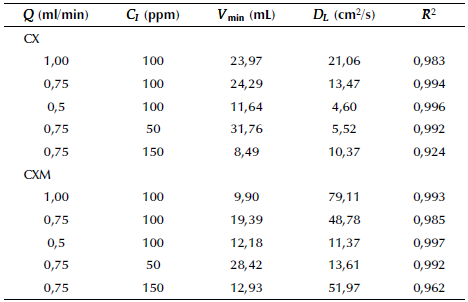
where Q (mL/min) is the feed flow rate, m (g) is the mass of the adsorbent, C i (mg/L) is the influent concentration, C e (mg/L) is the effluent concentration and t(min) is the adsorption time. For comparison purposes, the breakthrough point was taken at 10% of the initial concentration. Additionally, breakthrough curves were fitted with the axial dispersion model (Eq. 8), which assumes a dispersed plug flow through the bed characterized by an axial dispersion coefficient.
where erf(x) is the error function of x, u is the interstitial velocity of the fluid, D L is the axial dispersion coefficient, V is the volume of the fluid sent to the packed-bed in an elapsed time, Vmin is the minimum volume required to saturate the bed and L is the bed length. The model parameters DL and Vmin were determined by nonlinear regression analysis (Arango Cardenas, 2015).
Results and Discussion
Characterization of TiO 2 nanoparticles and carbon xerogels
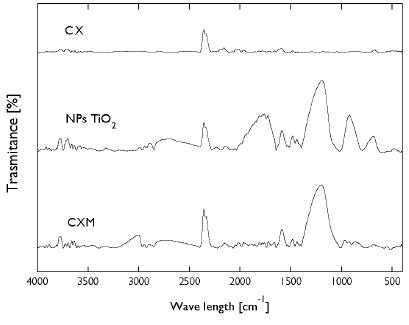
FTIR analysis was used to identify functional organic groups on the surface of carbon xerogels and TiO2 nanoparticles. The FTIR spectra are shown in Figure 1. CX shows peaks between 3600-3200 cm-1 due to the O-H stretching vibrations, which is characteristic of the presence of surface hydroxylic groups and chemisorption of water. Vibrations of the ring C=C=O between 2183-2160 cm-1 and 1751-1581 cm-1 were also observed, which can be attributed to the stretching vibrations of C=O moieties in carboxylic structures. The band at 1700 cm-1 can be related to the stretching vibrations of C=O from an unreacted aldehyde group and/or to the carbonyl generated when formaldehyde undergoes a ring-opening reaction (/Álvarez et al., 2015). The bands near 1473 and 1435 cm-1 were assigned to the C-H stretching vibrations in organic structures, Moreover, bands at 1300-1000 cm-1 and 933-920 cm-1 can be related to the C-O-C vibrations of methylether bridges in the resorcinol molecules and tri-substituted benzene rings, respectively (Girgis, El-Sherif, Attia, and Fathy, 2012; Rodrigues et al., 2012; /Álvarez et al., 2015).
Source: Authors
Figure 1 FTIR analysis for carbon xerogel, TiO2 nanoparticle and carbon xerogel modified.
For carbon xerogel modified with TiO2 nanoparticles, a peak at 3695 cm-1 was observed (Fig. 1). This band can be attributed to the Ti-COOH between the nanoparticles and carbon material. Bands between 2978 and 2870 cm-1 correspond to the presence of alkenes, carboxylic acids, secondary amines and phenols, whereas bands between 1188-910 and 1 581 cm-1 are associated with the presence of the Ti-O-C bond. Likewise, bands between 1728-1600 cm-1 correspond to the vibrations of stretching and flexion of hydroxyl groups bound to titanium Ti-OH atoms, whereas between 1489-1435 cm-1 are bands corresponding to CH3 deformation (Bitar Castro and Mejía Meza, 2015; Fornaris Lozada, 2015; Hudlikar, Joglekar, Dhaygude, and Kodam, 2012; Santhoshkumar et al., 2014). CXM shows peaks between 2877-2401 cm-1 and 686 cm-1 corresponding to the presence of alkenes, C-N and C-H groups. Bands at 2360 cm-1 and 2993 cm-1 are related to the presence of carboxylic acids and aromatic or aliphatic C-H groups. Bands at 3780, 1589 cm-1, 1481-1422 cm-1, and 1094-779 cm-1, are associated to hydroxyl group adsorbed in the sample, the presence of Ti-O-C group and the CH3 deformation, respectively. These peaks demonstrate that the carbon xerogel was effectively modified with TiO2 nanoparticles.
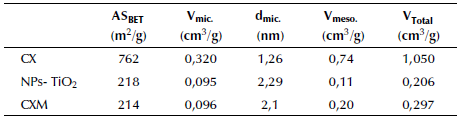
Since carbon xerogels are mesoporous materials, Table 1 shows values about texture and porous structure of the carbonaceous materials considered in this study. Values of SBET and porous parameters are higher than those obtained by Álvarez et al. (2015) for carbon xerogels, which could be due to the physical activation of the material. However, the surface area is similar to the one found for activated carbons, that is, between 824 and 1 237 m2/g (Paez et al., 2012). Likewise, the surface area for TiO2 nanoparticles agrees with those reported in literature of 116-586 m2/g (Bailón-García et al., 2017; Zhou, Zhang, and Liu, 2012). Additionally, the lower SBET for the modified materials is attributed to the presence of TiO2 nanoparticles, since microporosity is mainly associated with the carbon phase (Bailon-Garcia et al., 2017).
Table 1 Textural and porous parameters for carbon xerogel, TiO2 nanoparticle and carbon xerogel modified with nanoparticles
Source: Authors
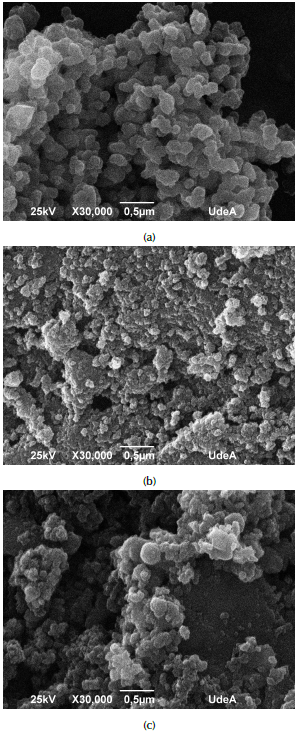
Figure 2 shows the scanning electron micrographs (SEM) of carbon xerogel, TiO2 nanoparticles and modified carbon xerogel. It is observed that nanoparticles are agglomerated, with a rounded structure and different sizes (Figure 2a). Activated carbon xerogel exhibits a smooth surface with a non-homogeneous particle size distribution, in addition to the formation of macropores, where TiO2 nanoparticles can be deposited and ASA molecules adsorbed in the removal process (Figure 2b). For the modified carbon xerogel (Figure 2c), the micrographs show TiO2 nanoparticles uniformly distributed on the surface of the material. These results agree with some previous studies, such as those by Álvarez et al. (2015), and Herrera, Reyes and Colina-Márquez (2016), where SEM images exhibit similar morphologies.
Source: Authors
Figure 2 SEM-EDS results for a) nanoparticles of TiO2; b) carbon xerogel; c) carbon xerogel modified with nanoparticles.
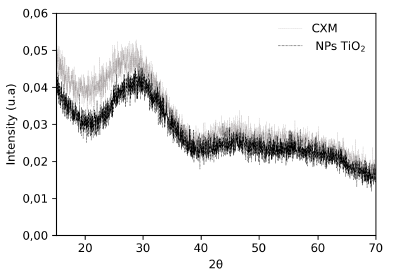
The XRD technique allows the identification of the phases present in a sample and their degree of crystallinity. Figure 3 shows the results of XRD forTiO2 nanoparticle and carbon xerogel modified. Peaks at 2θ = 25°, 30°, 48°, 50°, 54°, 55° and 62° correspond to the anatase phase, while peaks at 44° and 64° can be related to the rutile phase. The polymorph anatase form has a metastable characteristic, it is the most photoactive form of TiO2, and it is widely studied due to their technological importance in various applications (Keane, 2013).
Batch experiments
Dose curve and pH effect: The dose curve (Figure 4) shows an increase in ASA removal as the amount of CX increases, until reaching a maximum removal percentage of 91,82 % for an optimal dosage of 4,0 g/L.
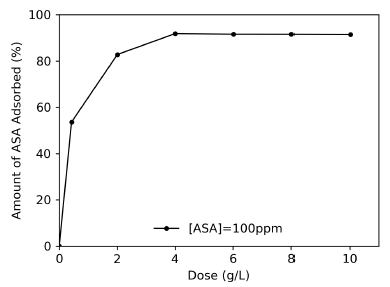
Source: Authors
Figure 4 Effect of carbon xerogel dosage on the adsorption of ASA. Initial concentration = 100 mg/L; solution volume = 25 mL; time = 7 h; agitation speed = 150 rpm.
The effect of the solution pH on the adsorption of ASA by CX is shown in Figure 5. The solution pH is a significant parameter that affects the adsorption process, as it alters the degree of ionization of the functional groups on the carbon surface. The adsorption capacity decreases with an increase in the solution pH. It has been suggested by Beninati, Semeraro, and Mastragostino (2008) that at low values of pH, the surface of the CX is positively charged, which produces an attractive force of the ASA anion, considering phenomena such as chemical bond, π - π -type interactions, and electrostatic interactions related to the amphoteric nature of the carbons. As the pH of the ASA solution increases, a proportional decrease in adsorption takes place, probably due to the successive deprotonation of positive charged groups on the adsorbent surface and a subsequent electrostatic repulsion between ASA and the negatively charged sites on the adsorbent. There also exists a competition between OH (at high pH) and ASA for the remaining positively charged adsorption sites (Mphahlele, Onyango, and Mhlanga, 2015).
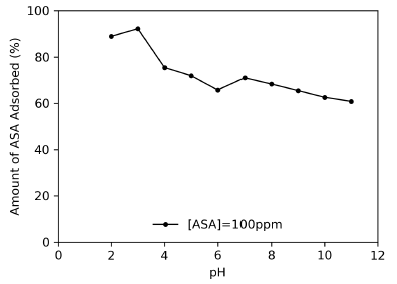
Source: Authors
Figure 5 Effect of the solution pH on the adsorption of ASA. Initial concentration = 100 mg/L; solution volume = 25 mL; time = 7 h; agitation speed = 150 rpm.
Adsorption isotherms: The adsorption isotherm describes the equilibrium relationship between bulk activity of the adsorbate in solution and adsorbed on the surface of the adsorbent at constant temperature (Pal, Deb, Deshmukh, and Verma, 2013). Figure 6 shows adsorption isotherms of ASA on carbon xerogel and carbon xerogel modified with TiO2 nanoparticles. According to the classification system proposed by Giles, MacEwan, Nakhwa and Smith (1960), the isotherms for CX (Figure 6a) and CXM (Figure 6b) would correspond to the S class sub-group IV, given the initial slope and the shape of the upper part of the curves. This type of isotherms is typical for mesoporous materials, which indicates that adsorption becomes easier as ASA concentration raises. This also evidence that water molecules contribute negatively to adsorption, as they compete with ASA for active sites on surface. Likewise, the curve appears in sub-group IV, which is characterized by the development of a fresh surface where adsorption can occur, probably due to re-orientation of molecules already adsorbed (/Álvarez et al., 2015; Giles et al., 1960).
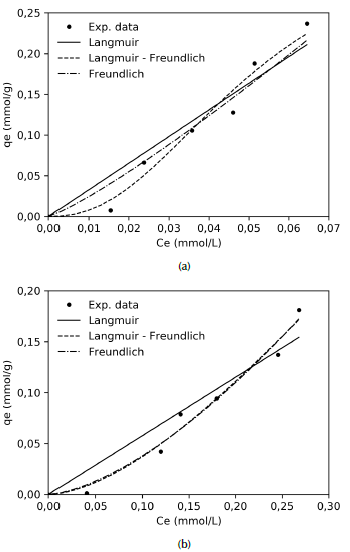
Source: Authors
Figure 6 Adsorption isotherm of ASA onto (a) carbon xerogel and (b) modified carbon xerogel. C0 = 10-200 mg/L; xerogel dose = 4 g/L solution volume = 25 mL; pH = 3; T = 30 °C; t = 2 h.
Table 2 shows the fitting parameters of the Langmuir, Freundlich and Langmuir-Freundlich models (Equations 3-5) to experimental data. According to the correlation coefficient R2, the adsorption of ASA onto CX and CXM is better fitted by the Langmuir-Freundlich model. This type of isotherm considers the heterogeneity of the solid surface, and is one of the models that best represents the removal of emerging contaminants (Álvarez et al., 2015).
Table 2 Parameters of Langmuir, Langmuir-Freundlich (L-F) and Freundlich models for ASA
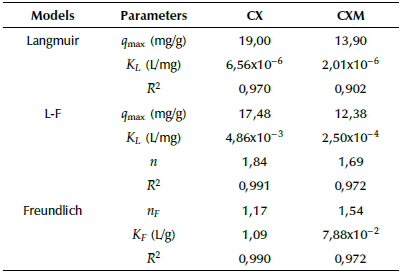
Source: Authors
The maximum adsorption capacity (qmax) for CX and CXM was 17,5 mg/g and 12,4 mg/g, respectively. The lower adsorption capacity of CXM may be due to the micro- and meso-porosity blockage by TiO2 nanoparticles, which is confirmed from results of porous parameters (Table 1). This fact denotes a preferential adsorption of ASA on the carbon surface, as found by Bailon-Garcia et al. (2017) for the adsorption of orange G dye on TiO2-carbon xerogel composites. Some of the functional groups of ASA that can occupy the available active sites on the surfaces of CX and CXM are aromatic rings, through a charge transfer, and groups -OH and -OOH, by means of electron donation (Rakic, Rajic, Dakovic, and Auroux, 2013).
Continuous experiments
Breakthrough curve of acetylsalicylic acid: Results for the breakthrough curves at different feed conditions are shown in Figures 7 and 8. On the one hand, Figure 7 evidence that increasing the ASA feed concentration makes the bed saturation process faster. This fact may be attributed to an increase of the driving force for the mass transfer of the adsorbate from the bulk of the fluid phase to the solid phase, enhancing the adsorption rate. However, the microcolumn packed with CXM (Figure 7b) saturates sooner than the one packed with CX (Figure 7a) for the same concentration feed. This is likely due to the presence of TiO2 nanoparticles that hinder the adsorption of ASA molecules, as was exposed in the batch experiments. Furthermore, the breakthrough curve for CXM shows a rather slow approach toward C/Co = 1 asymptote, which is a well-known characteristic of particle-controlled systems. This fact is a consequence of the enhanced external mass transfer exhibited by microdevices and the low porosity of the material.
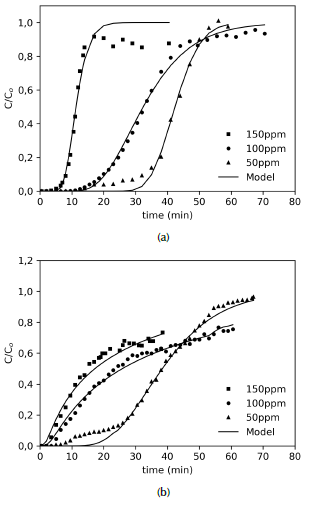
Source: Authors
Figure 7 Breakthrough curves for ASA adsorption on (a) CX and (b) CXM for different feed concentrations. Co = 50, 100, 150 mg/L; Q = 0,75 mL/min.
On the other hand, Figure 8 shows that increasing the feed flow rate leads to a steeper breakthrough curve, since contact time between solution and solid is too short, resulting in a reduction in the breakthrough time. In this case, the solute has not enough time to interact with the surface of the adsorbent and diffuse into the pores, particularly with large molecules such as those of organic compounds, thus adsorption is limited (Cabrera-Lafaurie, Roman, and Hernández-Maldonado, 2015). This behavior is more evident in the CXM sample (Figure 8b) than in the CX sample (Figure 8a), which could also be explained by the TiO2 blocking active sites.
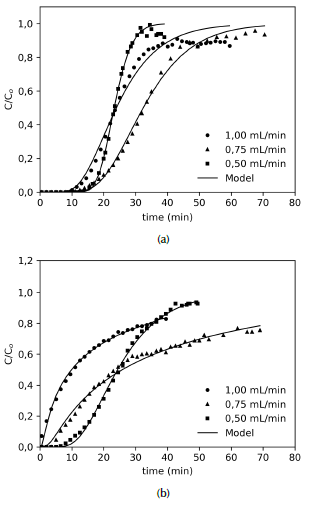
Source: Authors
Figure 8 Breakthrough curves for adsorption of ASA on (a) CX and (b) CXM for different volumetric flows. C 0 = 100 mg/L; Q = 0,5, 0,75 , 1,0 mL/min.
Table 3 summarizes the experimental results for break-throughtime and adsorption capacity of the microcolumns, as calculated from Equation 7. The adsorption capacity was compared with the results obtained from the adsorption isotherms, qmax. A significant decrease can be observed in < z the amount of ASA retained by the xerogels. This can be explained by the fact that during the batch experiments, the CX and CXM samples had more contact time with the ASA solution, in addition to the constant stirring. This favored the interaction with the active sites and enhanced the mass transfer rate.
Equation 8 was used to fit the experimental breakthrough data. A summary of the estimated parameters, Vmin and DL, is presented in Table 4. It is observed that in most cases the correlation coefficient R2 is higher than 0,99, indicating that an axial dispersion model is appropriate to describe well the breakthrough data. Furthermore, the axial dispersion coefficient increases with the volumetric flow rate in both adsorbents, obtaining in the sharpest breakthrough curves. Additionally, this indicates that there is an important contribution of the convection in flow direction. Finally, microcolumns packed with CXM exhibit higher values of DL, which demonstrates a poor retention of ASA on this material.
Conclusions
The removal of acetylsalicylic acid (ASA) was studied using carbon xerogel and carbon xerogel modified with TiO2 nanoparticles. It was found that low pH values favor the adsorption of ASA due to the positive charge of the carbon surface. The equilibrium experimental data for the CX and CXM were well fitted by the Langmuir-Freundlich model, where the maximum adsorption capacity of ASA was 17,48 mg/g and 12,38 mg/g for CX and CXM, respectively. Continuous experiments show that when increasing the inlet concentration and volumetric flow, breakthrough times are lower. In addition, the maximum adsorption capacities of the microcolumns were 8,42 mg/g for CX and 6,69 mg/g for CXM. Breakthrough curves agree well with the axial dispersion model. Results obtained from both equilibrium and continuous operation, demonstrate that the CXs could be used as a potential material to remove emerging contaminants such as ASA. Finally, despite experiments were not performed, the incorporation of TiO2 nanoparticles would allow the subsequent elimination of the contaminant using a process of photodegradation, when exposed to UV or visible light, as shown by many authors in previous works such as Bailón-García et al. (2017), Zheng et al. (2018) and Ge, Zhang and Park (2019).