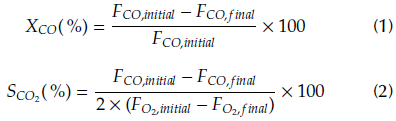Introduction
Proton Exchange Membrane Fuel Cells (PEMFC) are very interesting in portable applications, since they generate energy from hydrogen with high efficiency, reduced emissions and quick responses to charge changes (Salemme, Menna, Simeone and Volpicelli, 2010). The production of H2 for PEMFC is usually accomplished by multistep processes that include catalytic reforming of hydrocarbons or oxygenated hydrocarbons followed by water-gas shift (WGS) reaction (Martínez-Arias, Hungría and Múnuera, 2006). However, the gas stream obtained from these processes contains, in most cases, small amounts of CO and H2O. The PEMFC have low tolerance to CO (10 ppm), due to the poisoning by CO adsorption on the cell Pt anode. For this reason and for the effect on the kinetic of oxidation, there is a limitation to expand their use (Cheng et al., 2007; Choudhary and Goodman, 2002; Ko et al., 2006).
Several methods have been reported for carbon monoxide removal from the steam reforming stream, such as preferential oxidation of CO (CO-PROX), selective methanation of CO, and selective diffusion through membranes (Haryanto, Fernando, Murali and Adhikari, 2005; Manzolini and Tosti, 2008; Ocampo, Louis and Roger, 2009). Among these, preferential oxidation of CO (CO-PROX) is the simplest and most direct and accessible method for removing CO (Avgouropoulos et al., 2002; Epling, Cheekatamarla and Lane, 2003).
Cerium oxide (CeO2) has been broadly reported to be an active support and promotor for the synthesis of oxidation catalysts due to its structural properties (Fonseca, Royer, et al., 2012) and redox behavior that allows high oxygen mobility and storage, thus enhancing oxygen exchange with the reaction environment (Laguna et al., 2010; Trovalleri, 2002). Additionally, it has been found that the addition of ZrO2 improves the thermal stability and the redox properties of CeO2. It also improves oxygen mobility due to the formation of a mixed oxide system that promotes electronic distortions in the oxide framework, which contributes to the homogeneous dispersion of the deposited metal, long-term stability of the catalyst and an increase in the resistance to high temperatures, avoiding sintering problems (Biswas and Kunzru, 2008; Das et al., 2015).
In CO-PROX, gold supported catalysts in form of nanoparticles present better catalytic performance. Compared with other noble metals, Au catalysts are more active and selective for CO oxidation than for H2 oxidation (Choudhary and Goodman, 2002; Haruta, 2002). Thus, the high dispersion of nano-gold particles provides a suitable catalytic activity in CO oxidation (Haruta, Kobayashi, Sano and Yamada, 1987). Gold supported on cerium oxide catalyst has been studied for the CO-PROX process due to its capacity to store and provide oxygens in the reaction of CO oxidation (Fonseca, Royer, et al., 2012). However, this catalysts can present the drawback of poor resistance to the presence of CO2 in the reactant mixture (Trovalleri, 2002).
Recently, copper-based catalytic systems have emerged as promising substitutes for such noble metal catalysts, especially with CeO2 as support, thanks to their low cost, high catalytic performance and high selectivity of O2 to CO2 (W. Liu and Flytzaniste phanopoulos, 1995; Martínez-Arias et al., 2003). The excellent catalytic performance of CuO/CeO2 catalyst is on account of the high oxygen storage/release capability of CeO2 via the redox couple Ce4+/Ce3+ and the strong interaction between CuO and CeO2 (Kosmambetova et al., 2010). Mariño et al. (2008) concluded that the activity of copper and cerium oxides was lower when evaluated separately. However, the catalyst prepared with both oxides presented a high performance in the CO-PROX.
For CuO x /CeO2 catalysts, numerous preparation methods have been described in the literature, including co-precipitation, impregnation, inert gas condensation, and citric acid complexation-combustion (Di Benedetto, Landi and Lisi, 2018; Marbán and Fuertes, 2005; Zhu etal., 2017). In all cases, nano-sized crystals powders have been obtained.
The cooperative effect of the bimetallic gold and copper oxide has been studied to find an efficient catalyst in the CO-PROX, showing that bimetallic catalysts present better activity and selectivity than monometallic ones. Combining the catalytic properties of studied catalysts both metals has shown an intermediate behavior with good activity (98 % to 80 °C) and selectivity (65 % at 80 °C) (Fonseca, Ferreira, etal., 2012). Consequently, bimetallic gold-copper oxide supported catalyst has been studied in the CO-PROX reaction. Mozer, Dziuba, Vieira and Passos (2009) prepared the bimetallic Au-Cu catalyst on alumina and concluded that the addition of copper increased the selectivity to CO oxidation and reduced the H2 consumption. Liu, Wang, Zhang, Su and Mou (2011) studied bimetallic Au-Cu catalysts over silica and reported a higher catalytic activity than in monometallic catalysts. They also observed the presence of highly disperse nanoparticles on the support with significantly lower particle size. Li etal. (2012) evaluated bimetallic catalysts of gold and copper oxide over mesoporous silica Au-CuO/SBA-15. However, this catalyst is deactivated easily due to both ambient condition and high temperatures.
Laguna et al. (2012) studied catalysts CuO x /CeO2 and Au- CuO x /CeO2 (10 % w/w copper and 1 % w/w gold), which presented a better catalytic activity than monometallic catalysts of copper. In contrast, Reina, Ivanova, Laguna, Centeno and Odriozola (2016) concluded that the addition of gold (2 % w/w) in the system Cu/CeO2 with 15 % w/w of copper incorporated in the support do not promote the catalyst activity, due to the high load of the active phase.
Due to the complexity of some of the preparation methods above mentioned, it becomes necessary to search for simplest recipes directed to the potential mass-scale fabrication of the catalyst. The present study proposes to evaluate the cooperative effect between gold and copper oxide supported on cerium-zirconium mixed oxides in the CO-PROX reaction. Two bimetallic catalysts were prepared: one by incipient wetness impregnation to disperse copper over a previous gold supported on cerium-zirconium mixed oxide and another using copper incorporated in cerium-zirconium oxide support, where the addition of gold was made by co-precipitation.
Materials and methods
Catalyst preparation
Six catalytic materials were prepared to be evaluated in the CO-PROX reaction: (1) cerium-zirconium mixed oxide support (CeZr), (2) gold on cerium-zirconium mixed oxide (Au/CeZr), (3) impregnated copper on cerium-zirconium mixed oxide (CuO x /CeZr), (4) copper incorporated into cerium-zirconium mixed oxide (CuCeZr), (5) bimetallic catalyst Au-CuO x with impregnated copper oxide (Au- CuO x /CeZr) and (6) bimetallic catalyst Au- CuO x with incorporated copper (Au/CuCeZr).
The cerium-zirconium mixed oxide support (CeZr) and the copper incorporated into cerium-zirconium mixed oxide (CuCeZr) were prepared using the pseudo sol-gel like method (Vargas, Libs, Roger and Kiennemann, 2005), based on the thermal decomposition ofpropionate precursors. The starting materials were cerium (III) acetate hydrate (99,9 %, Sigma-Aldrich), zirconium (IV) acetylacetonate (98 %, Sigma-Aldrich) and copper (II) acetate monohydrate (99,5%, Sigma-Aldrich). The salts were dissolved separately in propionic acid (99,5 %, Sigma-Aldrich) under ebullition, mixed during 1 h, and then carried to controlled evaporation under vacuum pressure. The resin obtained was calcined at 550 °C for 6 hours.
The addition of gold was made following the methodology reported by Lin and Wan (2003). A suitable amount of tetrachloroauric acid trihydrate (> 99,9%, Sigma Aldrich) was dissolved in 250 ml of distillated water and then, a solution of sodium hydroxide (97,0%, Merck) was added for adjusting the pH solution to 6. After that, the support material (CeZr or CuCeZr) was mixed with the gold solution and stirred for six hours at ambient temperature. After filtration and washing, the solid was carried-out to calcination at 350 °C for 8 hours. The samples obtained were gold on cerium-zirconium mixed oxide (Au/CeZr) and bimetallic catalyst Au- CuO x with incorporated copper (Au/CuCeZr).
The monometallic and bimetallic catalysts impregnated with copper were prepared using incipient wetness impregnation method (Fonseca et al., 2012). Copper nitrate trihydrate (Chemi) was dissolved in distillated water, then added over the catalytic material (CeZr or Au/CeZr) with uniform mixing at ambient temperature. Finally, the mixture was carried to calcination at 350 °C for 8 hours. The samples obtained were impregnated copper oxide on cerium-zirconium mixed oxide (CuO x /CeZr) and bimetallic catalyst Au-CuO x over cerium-zirconium mixed oxide (Au- CuO x /CeZr).
Catalyst characterization
The specific surface area was determined from N2 adsorption/desorption isotherms using the BET method in an Autosorb-1 Quantachrome. The metallic composition was determined by atomic absorption spectrophotometry (GBC, AVANTA E) coupled to flame ionization. The Temperature Programmed Reduction (H2-TPR) was carried out with a ChemBET Pulsar TPR/TPD equipment from Quantachrome instruments following the H2 consumption by TCD. The textural morphologyofthe materials was studied by Scanning Electronic Microscopy (SEM) (JEOL, JSM-6490LV) and Transmission Electronic Microscopy (TEM) (Tecnai F20 Super Twin TMP of FEI). X-Ray Diffraction (XRD) was carried out with a PANalytical X'Pert PRO MRD equipment. Thermogravimetric Analysis (TGA) was performed using a TGA1-Mettler Toledo equipment.
Catalyst test
The catalytic tests of CO-PROX were carried out in a U-shaped reactor connected online to a gas chromatograph (Hewlett Packard 5890 Series A) with a packed molecular sieve 13X column, and a TCD at 0,76 atm pressure. The temperature was controlled using a cylindrical shape furnace with a thermocouple in the center of the system close to the catalytic fixed-bed. The samples were submitted to an oxidant atmosphere treatment for 1 h at 300 °C. The reaction gas feed consisted of 2% vol. CO, 2% vol. O2, 50%vol. H2 with He as gas balance, and a total flow rate of 100 mL/min, through 100 mg of the catalyst with 60-80 mesh. The corresponding space velocity is 60 000 mL g-1 h-1. The content of CO2 and H2O in the reaction stream was limited to 2 % vol. and 3 % vol., respectively, instead of 10-20 % vol. (real content), due to the experimental restrictions of the catalytic reaction system. However, this amount was enough to observe the kinetic inhibition effect by competitive adsorption in the CO-PROX reaction (Córdoba and Martínez-Hernández, 2015).
In this study, the catalytic test consisted of four steps. In step I, the bimetallic catalysts Au- CuO x /CeZr and Au/CuCeZr were evaluated in the CO-PROX and the best catalytic performance, Au- CuO x /CeZr, was compared with the monometallic catalysts Au/CeZr and CuO x /CeZr. In step II and III, the CO2 and H2O effect of Au-CuOx/CeZr bimetallic catalyst was evaluated, considering that both compounds can be produced in the reforming process for hydrogen production (Cobo et al., 2013; Tippawan and Arpornwichanop, 2014). Finally, in step IV, the catalytic stability test was performed on Au-CuOx/CeZr bimetallic catalyst for 118 h at the temperature with the major activity, with CO2 and water in the feed effluent.
The CO conversion (Xco) and selectivity to CO2 (SCO2) were calculated using Equation (1) and Equation (2), respectively.
where F CO,initial and F O2,initial are the molar flows of CO and O2 in the feed stream, and F CO,final and F O2,final are the molar flows in the outlet stream.
Results and discussion
N 2 adsorption-desorption isotherms
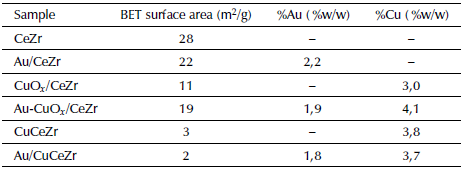
Table 1 presents the BET specific area and the composition percentages of the metals loaded in the catalytic materials. The quantified experimental values agree with the theoretical values (2 % and 4% w/w for gold and copper, respectively) indicating that the synthesis methods are suitable for obtaining reproducible catalytic materials.
Table 1 Chemical composition and BET specific area of supports, bimetallic and monometallic samples
Source: Authors
The isotherms for the bimetallic catalytic materials, which are not shown here, were type IV, typical of mesoporous materials (Thommes et al., 2015), with a H2 hysteresis, related to the not well-defined distribution of pore shape materials (Thommes, 2007).
It is evident that incorporated copper samples showed the lowest specific area, and that mono and bimetallic samples present a lower surface area than support materials. The inclusion of copper ions in Cerium-Zirconium mixed oxides modifies the crystalline structure and the presence of Au, CuOx and Au-CuOx facilitates the obstruction of the smaller porous, which results in a lower surface area compared with the supports (Gurbani et al., 2009).
In the case of Au-CuOx/CeZr bimetallic catalyst, the surface area is slightly lower than Au/CeZr sample but higher than CuO x /CeZr. This behavior can be related to a higher dispersion of the CuO x derived from the interaction with gold, avoiding the subsequent porous obstructions.
Temperature programmed reduction (TPR-H 2 )
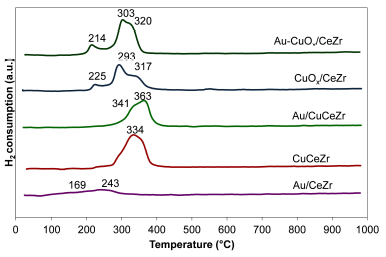
Figure 1 presents the TPR-H2 patterns of the supports and the catalytic samples. Due to the limitations of the TPR test, results were only analyzed qualitatively.
Source: Authors
Figure 1 TPR-H2 patterns of the bimetallic catalysts Au-CuOx/CeZr, Au/CuCeZr; the monometallic catalysts CuOx/CeZr, CuCeZr, Au/CeZr; and the CeZr support.
The reduction profile of Cerium-Zirconium support (not shown) is characterized by a wide peak at about 563 °C, which is attributed to the strong interaction between CeO2 and ZrO2 and the solid solution obtained (Córdoba and Martínez-Hernández, 2015).
The Au/CeZr reduction pattern shows two hydrogen consumption peaks around 170 and 240 °C, which are related to the reduction of Au3+ species, promoting slightly the H2 spillover phenomenon and therefore the cerium reduction at lower temperature (Fonseca et al., 2012; Laguna et al., 2014).
The TPR profiles for CuOx/CeZr and Au-CuOx/CeZr are similar, showing three reduction peaks (Figure 1) at around 220, 300 and 320 °C, indicating no strong effect of gold on the cerium reduction temperatures. The first peak correspond to the reduction of copper clusters with lower oxidation, the next peak to the reduction of isolated copper species with higher oxidation state and the last peak to the simultaneous reduction of copper and surface cerium oxide (Gurbani et al., 2009; Liao, Chu, Dai and Pitchon, 2013; Martínez-Arias et al., 2009).
The samples with incorporated copper (CuCeZr, Au/CeZr) present two reduction peaks at slightly higher temperatures than CeZr based samples, indicating a more difficult reduction for copper. The reduction peaks of incorporated copper samples can be attributed to the simultaneous reduction of highly dispersed species (Cu2+ to Cu1+) in strong interaction with the support, and the superficial Ce4+ reduction in the interface Cu-CeO2 (Araújo, Bellido, Bernardi, Assaf and Assaf, 2012; Reina et al., 2016).
Textural morphology
The morphology of the catalytic materials was evaluated by Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM). Figure 2 presents the SEM of the bimetallic catalysts.
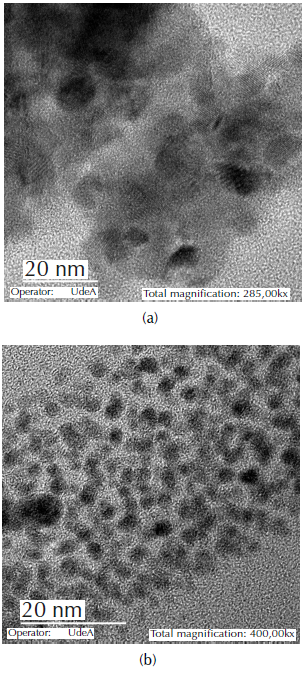
The SEM results show that the catalyst Au- CuO x /CeZr presented smaller particles formation on the larger particles of the material, while the Au/CuCeZr did not generate this type of particle. The formation of smaller particles can be attributed to the presence of CuOx on the bimetallic catalyst with impregnated copper (Au- CuO x /CeZr). Figure 3 presents the TEM results of the bimetallic catalysts.
The formation of circular particles was promoted in the bimetallic catalyst with impregnated copper (Au- CuO x /CeZr). However, the presence of well-defined nano-gold particles was not observed, possibly due to the masking effect of the copper oxide or the agglomeration of gold particles in the calcination step.
For the bimetallic catalyst with incorporated copper (Au/CuCeZr), particles of around 4 nm are observed. EDX analysis and a closer micrograph are required to confirm the chemical nature of the particles, since the one presented in Figure 3 does not allow an undoubted differentiation of gold, taking into account the nature of the support.
X-Ray Diffraction
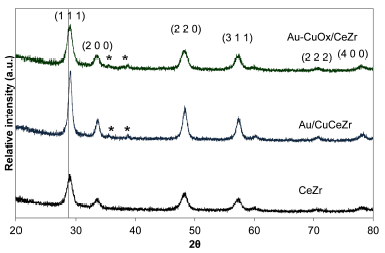
Figure 4 presented the XRD patterns for the bimetallic catalysts. The materials presented diffraction peaks corresponding to the fluorite cubic structure of the mixed oxide cerium-zirconium, which is identified by the planes (111), (2 0 0), (2 2 0), (2 2 0), and (4 0 0) (JCPDS Card No. 28-0271), with an incorporation of ZrO2 in the CeO2 lattice forming a homogenous solid solution (Biswas and Kunzru, 2007; Córdoba and Martínez-Hernández, 2015).
Source: Authors
Figure 4 XRD patterns of CeZr support and the catalysts Au-CuOx/CeZr and Au/CuCeZr *CuO.
Table 2 presents the average crystallite size and the lattice parameter (a) from XRD peak positions and indexation. The bimetallic catalysts did not present changes in the fluorite cubic crystalline structure when gold and copper are added. The position of peaks in both bimetallic catalysts shifted slightly toward high angles with respect to the support, which is in accordance with the decrease in the lattice parameter of bimetallic catalysts due to a possible interaction between the copper and the fluorite CeZr structure. This is not due to an insertion of copper in the crystalline structure forming a true solid solution, but to a high copper dispersion into the mixed oxides solution (Marban and Fuertes 2005).
Table 2 Structural parameters of the catalyst materials
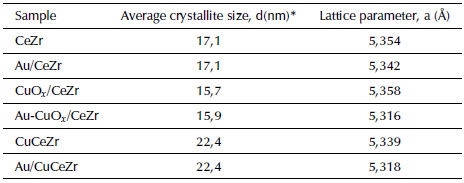
*Calculated with the Scherrer equation of the plane (111)
Source: Authors
Signals corresponding to gold did not present patterns in XRD in the bimetallic catalyst (Figure 4), since gold percentage (about 2 % w/w) is too low for being detected by XRD patterns. Additionally, it has been reported that gold particles below 5 nm cannot be detected by XRD (Ilieva et al., 2009; Reina, Ivanova, Centeno and Odriozola, 2014), which is in accordance with TEM results.
However, diffraction peaks 35,5° and 38,7° correspond to the cluster of copper oxide, such as tenorite (Moretti et al., 2015). These CuO signals were present in the bimetallic catalyst with impregnated copper (Au- CuO x /CeZr) and in the bimetallic catalyst with incorporated copper (Au/CuCeZr), discarding the formation of the solid solution Au-Cu (Laguna et al., 2014).
The TGA was done in the bimetallic catalyst with impregnated copper (Au- CuO x /CeZr) before and after CO-PROX stability reaction test to determine coke formation (not shown). Results did not show weight loss in this temperature range, which suggests that the bimetallic catalyst used Au-CuO x /CeZr present no carbonaceous deposits formation.
Catalytic test
• Step I
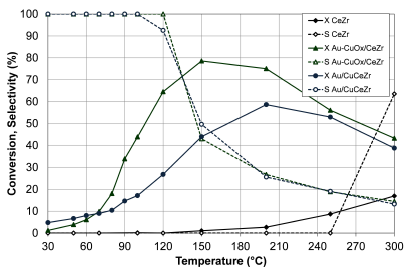
Figure 5 presents the results of the CO conversion and CO2 selectivity of both bimetallic catalysts.
Source: Authors
Figure 5 CO conversion (X, filled symbols) and selectivity to CO2 (S, empty symbols) of CeZr, Au-CuOx/CeZr and Au/CuCeZr. Feed stream: 2 % CO, 2 % O2, 50% H2 and He (balance).
The Au-CuOx/CeZr presented a better behavior with respect to the Au/CuCeZr due to the possible effect of copper in the CO-PROX activity. Cu-Ce interactions in the catalyst by wet impregnation promote the formation of large interfaces Cu-Ce, where CO is absorbed to oxidation. It is believed that the reaction is catalyzed by the interfacial copper oxide-ceria centers in which ceria presents a high number of oxygen vacancies that allows high mobility of lattice oxygen (Marbán and Fuertes, 2005).
The higher catalytic activity of the Au-CuOx/CeZr with respect to the Au/CuCeZr is in agreement with TPR-H2 results, where the Au- CuO x /CeZr presented a higher reducibility to lower temperatures than the Au/CuCeZr. Additionally, the Au/CuCeZr presented a lower surface area.
The catalytic behavior of the Au-CuOx/CeZr might be related to the formation of smaller particles on the large particles of the material (SEM results), as well as to the good size distribution of particles of about 5 nm (TEM results). The Au/CuCeZr favored the formation of nanoparticles of 4 nm (TEM results). However, the gold activity was inhibited with the incorporation of copper.
Figure 6 presents the CO conversion and selectivity to CO2 of the Au-CuOx/CeZr with respect to the monometallic catalyst of gold (Au/CeZr) and impregnated copper (CuOx/CeZr).
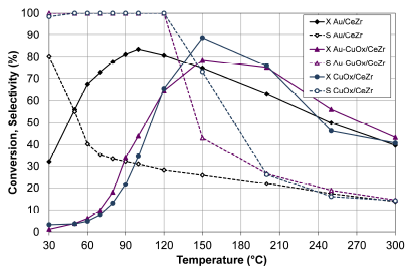
Source: Authors
Figure 6 CO conversion (X, filled symbols) and selectivity to CO2 (S, empty symbols) of Au-CuOx/CeZr with respect to Au/CeZr and CuOx/CeZr. Feed stream: 2 % CO, 2 % O2, 50% H2 and He (balance).
The bimetallic catalyst Au-CuOx/CeZr (Figure 6) showed the best catalytic performance, since the CO conversion curve volcano-type was wider than in the monometallic catalyst of gold (Au/CeZr) and impregnated copper (CuO x /CeZr). Although CuO x /CeZr had the highest CO conversion, the bimetallic catalyst provided greater stability throughout the evaluated temperatures. The good activity can be related to the bimetallic interaction, i.e. the formation of a cooper monolayer on gold plates, which allows the adsorption/activation of O2 and the formation of CO2 through CO and O coadsorption. This favored the formation of alloy active sites that participate in the CO oxidation and increase the reaction speed (Hussain, 2013). The selectivity of the Au- CuO x /CeZr was enhanced with respect to the monometallic catalyst Au/CeZr. To establish the bimetallic interaction, further experiments with techniques such as HR-TEM and XPS are needed.
The bimetallic catalyst of impregnated copper Au-CuOx/CeZr presented a better catalytic performance than Au/CuCeZr, therefore the Au-CuOx/CeZr was selected for the evaluation of the next step.
• Step II
Figure 7 presents the results of the CO conversion and the selectivity to CO2 of bimetallic catalyst with incorporated copper (Au-CuOx/CeZr), evaluating the effect of CO2 in the CO-PROX.
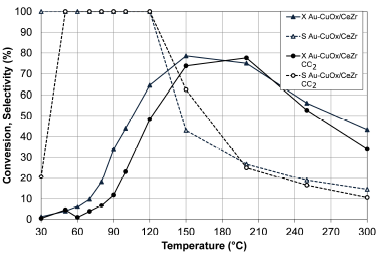
Source: Authors
Figure 7 Effect of CO2 on the CO conversion (X, filled symbols) and selectivity to CO2 (S, empty symbols) of Au-CuOx/CeZr. Feed stream: 2 % CO, 2 % O2, 2 % CO2, 50% H2 and He (balance). Step III
The addition of CO2 in the feed effluent affected the catalytic activity of Au-CuOx/CeZr, since it required a higher temperature to reach the maximum conversion (Figure 7). The bimetallic catalyst presented a slightly lower activity than the same catalyst evaluated without CO2. The selectivity was not affected by the CO2 addition. Laguna et al. (2014) reported that the presence of CO2 decreases CO conversion below 150 °C due to the competence in the adsorption between CO and CO2.
Figure 8 presents the results of CO conversion and selectivity to CO2 of Au- CuO x /CeZr, evaluating the effect of water and CO2 addition in the feed stream.
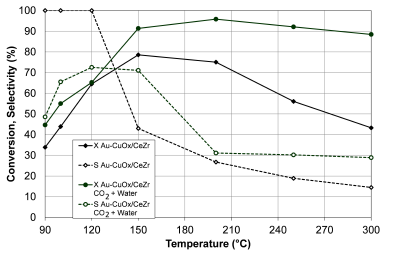
Source: Authors
Figure 8 Effect of water and CO2 on the CO conversion (X, filled symbols) and selectivity to CO2 (S, empty symbols) of Au- CuO x /CeZr. Feed stream: 2 % CO, 2 % O2, 2 % CO2, 3 % H2O, 50% H2 and He (balance).
The reaction feed mixture was humidified by bubbling it through a container of deionized water at room temperature, yielding 2 % water vapor in the gas fed to the reactor. The addition of water in the feed effluent had a positive effect on the bimetallic catalyst (Figure 8), increasing CO conversion with respect to bimetallic catalyst without CO2 and water. Au-CuOx/CeZr presented a conversion above 90 % between 150 and 300 °C. The catalyst achieved a maximum conversion of 96 % at 200 °C. The selectivity was better with water addition, even though it was inhibited below 120 °C.
The favorable effect of water on the activity of the bimetallic catalyst can be explained by the formation of hydroxide groups in the dissociative adsorption of water on the active sites of gold (Liao et al., 2013; Mozer et al., 2009). The presence of CO2 and H2O can also affect the catalytic behavior of the monometallic catalyst since a competitive adsorption is expected and therefore an effect on the activity and selectivity. Further studies are needed in order to confirm this effect.
• Step IV
Figure 9 presents the results of the catalytic stability of Au-CuOx/CeZr, evaluating CO conversion and selectivity to CO2 with the addition of water and CO2 in the feed effluent.
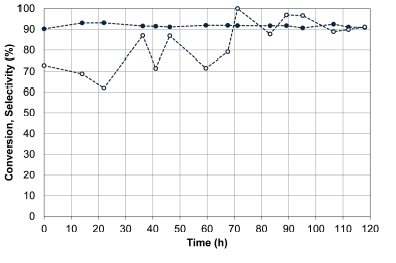
Figure 9 Catalytic stability of the Au- CuO x /CeZr. Evaluation of the CO conversion (X, filled symbols) and selectivity to CO2 (S, empty symbols) with addition of CO2 and water. Feed stream: 2 % CO, 2 % O2, 2 % CO2, 3 % H2O, 50% H2 and He (balance).
The bimetallic catalyst Au-CuOx/CeZr presented a good behavior in the stability test, with was stable for 118 h, obtaining CO conversion of 92% and selectivity of 90%, representing an ooutstanding performance. Fonseca, Ferreira et al. (2012)) evaluated a bimetallic catalyst Au-Cu over CeO2 in the presence of 10 vol. % of CO2 and 2 vol % of H2O, and reached a CO conversion of 60 % and a selectivity of 85 % during 8 h without deactivation. However, since the feed compositions evaluated in this work are quite different than those tested by Fonseca, Ferreira et al., the differences in the catalytic behavior could be related to the important role of these compounds.
Conclusions
All the samples presented the crystalline fluorite-type structure, with the presence of CuO in the monometallic and bimetallic materials with copper, enhancing the catalytic activity. It was possible to obtain nano-gold particles that promoted catalytic behavior.
The bimetallic catalyst with impregnated copper (Au- CuO x /CeZr) and the bimetallic catalyst with copper incorporated (Au/CuCeZr) were evaluated in the CO-PROX. Results showed that the bimetallic catalyst with impregnated copper presented the best catalytic performance, since it exhibited high activity, good selectivity and good long-term stability throughout the evaluated temperatures, even in the presence of CO2 and H2O in the feed stream.
The Au- CuO x /CeZr catalyst presents a cooperative effect between gold and copper, promoting higher stability in the catalytic activity, CO conversion and selectivity to CO2 in the evaluated temperature window. Au and Cu interaction promotes the redox properties of the CeZr mixed oxide allowing the reduction of Ce4+ ions to lower temperatures and improving the redox behavior.