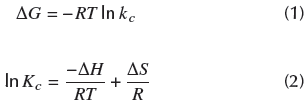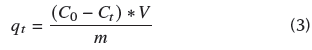Introduction
Chromium and its compounds are toxic when introduced in natural water from a variety of industrial wastes. These materials accumulate in the food chain and affect living organisms in the ecosystem (Jain et al., 2018; Carreño-Sagayo, 2016). The primary sources come from dyeing, canning, electroplating, leather tanning, metal processing, paints and pigments, and the textile and steel manufacturing industries (Akram, Bhatti, Iqbal, Noreen, and Sadaf, 2017; Vargas-Niño, Carriazo, and Castillo, 2011). Contamination in water currents by Cr(VI) represents a threat to animal and human health, as it can cause lung cancer, ulcers, perforations of the nasal septum, and kidney damage (Razi, Al-Gheethi, and Za, 2018). Due to such mutagenic and carcinogenic properties in living tissues, Chromium is one of the most harmful metals. It is included in the priority list of hazardous substances of the World Health Organization (WHO), with permissible limits of 0,05 ppm and 0,1 ppm established in drinking and inland water, respectively (Jain et al., 2018; Haroon et al., 2016).
Traditional methods of contaminated effluent treatment with Cr(VI) consist of chemical precipitation, filtration, oxidation and chemical reduction, electrocoagulation, reverse osmosis, ultrafiltration, ion exchange, adsorption, evaporation, among others (Razi et al., 2018; Rico et al., 2018; Akram et al., 2016). However, these processes have some limitations due to high energy consumption and large amounts of input chemicals (Wassie and Srivastava, 2016), whereas bio-adsorption is a versatile and effective method to remove heavy metals because it involves the use of low cost and high-efficiency adsorbents. In recent years, several low-cost materials have been reported, such as rice husk (Brahmaiah, Spurthi, Chandrika, Ramanaiah, and Prasad, 2015; Lin et al., 2018), moringa seeds (Maina et al., 2016), walnut husk (Casarin et al., 2016), orange peels (Giza, 2017; Abdelhafez and Li, 2016), among others. These lignocellulosic materials are suitable heavy metal adsorbents due to the presence of polymeric groups that serve as active centers for the uptake of metals (Marimón-Bolívar, Tejeda-Benítez, and Herrera, 2018).
To understand the phenomenon of heavy metal bio-adsorption in solutions, isotherms, kinetics, and desorption have been used. All of them provide information on the amount of ions adsorbed by a given biomass, the interaction mechanisms between adsorbents and adsorbate, adsorption mechanisms, among others, which enables the optimization of adsorption mechanism pathways, the expression of surface properties and adsorbent capacities, and the production design ofadsorption systems (Anastopoulos and Kyzas, 2016). It was found that the adsorption kinetics of Cr(VI) at different temperature values on oil palm bagasse was adjusted to the pseudo-second order model. The case of plantain peels was described by the Elovich model; for this reason, we can infer that the process is controlled by chemical adsorption (Villabona-Ortíz, Tejada-Tovar, and Ortega-Toro, 2020).
The study of adsorption thermodynamics allows us to identify the adsorption mechanisms involved in the process.
This is due to the fact that there are three main steps during the adsorption of a compound to the adsorbent surface. In the first step, molecular mass is transferred from the solution to the adsorbing surface. Then, internal molecular diffusion to the adsorption sites placed in the adsorbent takes place. Adsorption is completed in the final step of the process by attraction between adsorbate and adsorbent, either by physiadsorption or chemosorption (Kecili and Hussain, 2018). Consequently, studies for the modification of thermodynamic parameters are the focus of evaluations of adsorbent efficiency, in order to establish adsorption mechanisms and optimize the process. The most common equations that relate thermodynamic parameters are the change in Gibbs free energy (ΔG0), enthalpy (ΔH 0), and entropy (ΔS 0) in steady state (Tran, You, Hosseini-Bandegharaei, and Chao, 2017). As was reported previously, Cr(VI) adsorption by using coconut shells is endothermic, spontaneous, and irreversible (Ijeamaka, Christian, Fabian, MaryJane, and Joseph, 2018) and tamarind shells behave similarly (Bangaraiah, 2020). By contrast, adsorption bymeans ofcorn residues is reversible, favourable, spontaneous, and endothermic (Núñez-Zarur, Tejada-Tovar, Villabona-Ortíz, Acevedo, and Tejada-Tovar, 2018). Thus, the objective of the present work is to determine the influence of temperature on the adsorption of Cr(VI) present in aqueous solutions, using waste materials from the agro-industry such as palm bagasse and plantain peels. The thermodynamic parameters were calculated to establish the mechanisms that control the process.
Experimental
Biomass Preparation
The biomass, consisting of plantain peels and palm bagasse, was supplied by local businesses from the department of Bolívar, Colombia, where the biomass of interest was a residue of their production processes. It was prepared by applying a series of unit operations to adapt it to the necessary conditions for the bio-adsorption process. Firstly, the biomass was washed with deionized water to remove dirt and any pulp debris it could contain. Then, a size reduction was performed to facilitate its handling in later stages. A final wash with distilled water was carried out to remove tannins, resins or other compounds that could affect the process. The biomass was dried in an oven for 8 h at 60 °C, and then grounded and sieved until a particle size of 0,5 mm was obtained (Chieregato and Tapia, 2016). The bio-adsorbents were stored dry in airtight bags to keep them in good condition.
Solution Preparation
The solution was synthesized from 0,28288 g of potassium dichromate (K2Cr2O7) per liter of water deionized for the Cr (VI) solution, thus ensuring a concentration of 100 ppm. The solutions were brought to a pH of 2 with 2 M hydrochloric acid and sodium hydroxide (Tejada, Quiñones, Tejeda, and Marimón, 2015).
Thermodynamic parameters
For the calculation of thermodynamic parameters, Cr(VI) adsorption tests were carried out in a 100 mL Erlenmeyer shaker, simulating a batch reactor with a stirring of 200 rpm for 24 hours at a constant temperature. After 24 hours, the samples were removed from the agitator, and then the final concentration measurements of the metal were made. These data were determined with a UV-VIS spectrophotometer of the brand Shimadzu, model UV 17 000. The standard method to determine the amount of Chromium in water was applied using 1,5-diphenylcarbazide at 540 nm (Tejada-Tovar, Herrera-Barros, and Villabona-Ortíz, 2020).
The effect of temperature on Cr(VI) adsorption was determined by calculating Gibbs free energy (ΔG°), enthalpy (ΔH°), and entropy change (ΔS°), using Equations 1 and 2. The temperature values of 303,15, 328,15 and 352,15 K were taken with a particle size of 0,5 mm and a biomass quantity of 0,325 g.
The total adsorption capacity qt for each temperature was
initially calculated by using Equation 3
where C 0 (mg/L) is the initial concentration of the metal, C t (mg/L) is the metal concentration in the liquid phase at a time f, m is the mass of adsorbent used in (g), and V, the volume of the solution in (L).
Finally, the values of q for the different temperatures were obtained to perform the calculations of the adsorption equilibrium constants K c described in Equation (4). The van't Hoff graphic method was used on the obtained data to determine the change in Gibbs free energy (ΔG°) from the enthalpy change (ΔH°) and entropy change (ΔS°).
where C ac is the concentration of theadsorbate in equilibrium, contained in the surface of the adsorbent, C se is the concentration in solution in the equilibrium, and R is the universal constant of the gases (8,314 J/mol.K).
Results and discussion
Adsorbent characterization
From the Brunauer-Emmett-Teller (BET) analysis, it was found that the plantain peel has a larger surface area: 3,0889 m2/g compared to 2,7317 m2/g of palm bagasse. However, lignocellulosic residues are expected to have a low surface area due to their cellulose and hemicellulose content (Asuquo, Martin, Nzerem, Siperstein, and Fan, 2017). As for the pore size, value: 16,410 nm compared to 6,4457 nm of plantain peels, which indicates that both are mesoporous materials, making them suitable for adsorption in their liquid phase, since this facilitates Cr (VI) diffusion into the adsorbent structure (Hubbe, Azizian, and Douven, 2019).
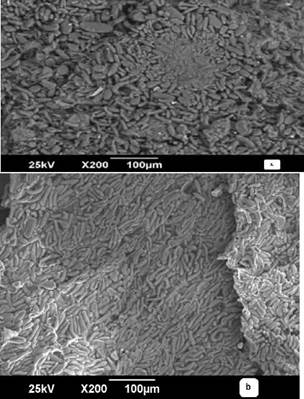
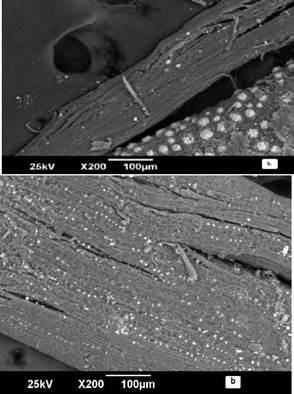
Figures 1 (a) and 2 (b) show the SEM micrographs. For the plantain peel, a cylindrical aspect is observed. In contrast, an entanglement structure with porosity is noted for the palm bagasse, typical of lignocellulosic materials (Martínez-Mendoza et al., 2020). This characteristic is extremely beneficial for metal ion transport which increases the adsorption capacity (Pradhan, Arora, and Mahajani, 2018).
In Figure 1 (b), the banana peel is shown after the adsorption process, where the cylindrical surface of the adsorbent was seen to be covered by Cr(VI) ions, manifested by white micro precipitated particles on the surface. Therefore, when Cr(VI) is captured, the ion adsorption mechanism in biomaterials is produced by the ion exchange between the metals under study and the active centers of the material. (Rodriguez-Narvaez, Peralta-Hernandez, Goonetilleke, and Bandala, 2017; Medellín-Castillo, 2017; Chen, An, Sun, Gao, and Qian, 2018).
After carrying out the Cr(VI) adsorption test using plantain peels and oil palm bagasse as bio-adsorbent, the effect of temperature, particle size, and amount of metal adsorbent were evaluated by calculating the capacity of Cr(VI) adsorption. Table 1 summarizes the results for each of the studied conditions. It was experimentally established that, at 55 °C, 0,5 mm, and 0,03 g of biomaterial, the highest adsorption capacity is obtained by using both bio-adsorbents. The results are 110,89 mg/g when using plantain peels, and 325,88 mg/g for palm bagasse oil can.
Table 1 The adsorption capacity of Cr (VI) on plantain peels and oil palm bagasse
| Temperature (°C) | Particle size (mm) | Mass of adsorbent(g) | Plantain peel | Palm bagasse |
|---|---|---|---|---|
| 40,0 | 0,355 | 0,15 | 52,93 | 38,36 |
| 70,0 | 1,0 | 0,15 | 66,24 | 15,82 |
| 40,0 | 1,0 | 0,5 | 19,95 | 18,01 |
| 55,0 | 0,5 | 0,62 | 15,99 | 14,89 |
| 55,0 | 0,13 | 0,325 | 30,46 | 7,15 |
| 80,2269 | 0,5 | 0,325 | 30,46 | 30,77 |
| 55,0 | 1,22 | 0,325 | 30,46 | 30,77 |
| 29,7731 | 0,5 | 0,325 | 30,46 | 25,22 |
| 55,0 | 0,5 | 0,03 | 110,89 | 325,88 |
| 70,0 | 0,355 | 0,15 | 66,24 | 66,67 |
| 40,0 | 1,0 | 0,15 | 53,11 | 34,21 |
| 55,0 | 0,5 | 0,325 | 30,46 | 29,48 |
| 40,0 | 0,355 | 0,5 | 20 | 19,99 |
| 70,0 | 1,0 | 0,5 | 19,88 | 4,69 |
| 70,0 | 0,355 | 0,5 | 19,90 | 19,99 |
| 55,0 | 0,5 | 0,325 | 30,61 | 29,48 |
Source: Authors
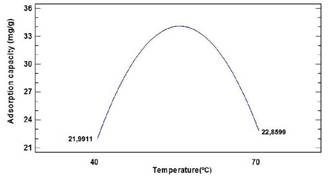
Figure 3 initially shows a gradual increase in adsorption capacity with an increase in temperature. This is due to the increase in interactions between adsorbate and adsorbent.However, at high temperatures, its effect is diminished (Sakulthaew, Chokejaroenrat, Poapolathep, Satapanajaru, and Poapolathep, 2017), with a noticeable change at 55 °C.
The reason for the descending behaviour of Cr(VI) removal at a high temperature and the immediate adsorption without positive effect due to its increase is that the metal ions in the solution could interact more with the binding sites at a lower temperature. As the temperature increases, so does the mobility of the ions, and the attractive forces between the sorbent and the metal ions decrease, thus decreasing the sorption efficiency of the adsorbent (Abbas et al., 2017). This behaviour suggests that the system is spontaneous and endothermic up to the evaluated intermediate temperature (55 °C). Also, when the temperature tends to higher values, the process becomes unfeasible under these conditions (Ajmani, Shahnaz, Subbiah, and Narayanasamy, 2019).
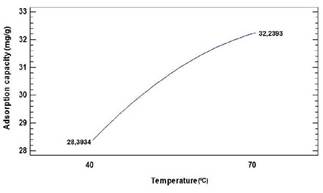
Figure 4 exhibits an increasing trend in adsorption capacity with an increase in temperature, which could be due to the chemical interaction between adsorbate and adsorbent, and the subsequent creation of new adsorption sites (Jain et al., 2018).
The possibility of presenting a higher diffusion rate of chromium ions in the shells is due to the formation of bonds between the ions and the active functional groups on the adsorbent. This overcomes the activation energy barrier and improves the rate of intraparticle diffusion, which can also interfere with the process (Aksu and Kabasakal, 2004) and increases the collision sequences between the adsorbate and the bio-adsorbent. Therefore, a more efficient adsorption and greater content of Cr(VI) ions are obtained in the shells (Soniyaand Krishnakumar, 2015). This behaviour coincides with the values reported when using coconut shell; it was found that an increase in temperature between 50 °C and 90 °C benefits the Cr(VI) removal process. This phenomenon is due to its endothermic nature and the increase in the kinetic rate of adsorption (Ijeamaka et al., 2018).
Enthalpy values of adsorption ΔH°, adsorption entropy ΔS°, and Gibbs energy ΔG° were calculated to establish the type of Cr(VI) adsorption by the studied materials. They allow setting the favourable process and the effect that temperature has on it (Figueroa, Moreno and Hormanza, 2014).
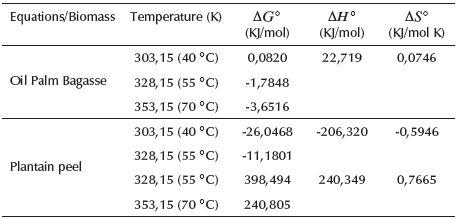
From Table 2, it was established that the process of Cr(VI) adsorption with oil palm bagasse becomes more favorable at higher temperatures, as it is a spontaneous process. Likewise, the enthalpy change indicates that the adsorption is endothermic and has a physical adsorption mechanism.
Table 2 Thermodynamic parameters for (VI) adsorption in a batch system using oil palm bagasse and plantain peels
Source: Authors
Besides, the positive value in entropy reveals the increase in randomness in the solid-liquid interface and evinces the high affinity of Cr(VI) ions with the residual material, as well as the probability of some structural changes due to the formation of Cr(VI) links -functional groups of bagasse in the interface. This value also hints to the possible reversibility of the process, which suggests that Cr(VI) ions replace some water molecules previously adsorbed on the adsorbent surface. These replaced water molecules reach a higher entropy by the translation of Cr(VI) ions, which allows the prevalence of randomness (Bedin, Martins, Cazetta, Pezoti, and Almeida, 2016; Jaiswal, Mani, Banerjee, Gautam, and Chattopadhyaya, 2015; Mthombeni, Onyango, and Aoyi, 2015). Similar values of enthalpy and entropy in the adsorption process were determined and recorded in the study on Cr(VI) adsorption by modified rice straw (Lin et al., 2018).
On the other hand, the positive values of the change of Gibbs free energy (ΔG°) in adsorption with plantain peels indicate that the process is thermodynamically feasible (spontaneous) at temperatures from 303,15 to 328,15 K and unfavorable (not spontaneous) at temperatures from 328,15 to 353,15 K (Khan, Nazir, Ali, and Kumar, 2017). Regarding the enthalpy values for the different stages of the Cr(VI) removal process, the negative value indicates that the process is exothermic in nature. In contrast, the positive value reveals the endothermal nature at temperatures below 353,15 K. It also suggests that the adsorption mechanism was physicochemical (Khan et al., 2017; Yang, Yu, and Qiu, 2014).
Conclusions
The effect of temperature on the Cr (VI) adsorption process was studied using plantain peels and oil palm bagasse as adsorbents, by determining the thermodynamic adsorption parameters and their physical significance on metallic removal. It was found that the biomaterials have a fibrous and cylindrical structure after the elimination of the ion micro-precipitates. It was determined that the process that controls the adsorption is the ion exchange between the adsorbent and the solution. The increase in temperature had a significant incidence on the removal, as it favors the adsorption capacity of the metal. From the thermodynamic parameters, it was determined that the adsorbents have a high affinity for Cr(VI) ions. The process is spontaneous up to 328,15 K. The removal is reversible on palm bagasse, exothermic up to 328,15 K on plantain peels and endothermic at higher temperatures. From the high adsorption capacities, these two materials are suggested as Cr(VI) adsorbents in aqueous solution.