Introduction
Quinoa (Chenopodium quinoa Wild) is an endemic South American plant typical, domesticated thousands of years ago by the Inca people of the Andes of Peru and Bolivia, as well as parts of Ecuador, Argentina, and Chile. It comes with a diversity of forms, genotypes, and wild progenitors (Navruz-Varli, and Sanlier, 2016), as well as a high resistance and adaptability to climatic and edaphic conditions (Jacobsen, Mujica, and Jensen, 2003; Filho et al., 2015).
Quinoa grain is considered to be a pseudocereal with a high nutritional value, mainly due to its high protein content and essential amino acids, which are higher than traditionally consumed cereals (Filho et al., 2015; Dakhili, Abdolalizadeh, Hosseini, Shojaee-Aliabadi, and Mirmoghtadaie, 2019), whose importance is increasingly recognized in food safety, since it considerably replaces and complements the diet, especially for people who rarely consume meat and dairy products (Elsohaimy, Refaay, and Zaytoun, 2015; Navruz-Varli, and Sanlier, 2016). It is one of the main sources of protein in developing countries (Repo-Carrasco-Valencia, and Serna, 2011).
Quinoa grains have been found to contain numerous phytochemical compounds such as phytosterols, phytoecdysteroids, and phenols, which provide health benefits with their metabolic, cardiovascular, gastrointestinal, and anticarcinogenic aspects (Navruz-Varli and Sanlier, 2016; Vilcacundo and Hernández-Ledesma, 2017), and therefore hav a high antioxidant activity, especially associated with phenolic compounds (Contreras-Jiménez, Torres-Vargas, and Rodríguez-García, 2019; Repo-Carrasco-Valencia, 2020), with up to 27 types of free and conjugated phenols (Abderrahim, Huanatico, Segura, Arribas, Gonzalez, Condezo-Hoyos, 2015), mostly phenolic acids (Repo-Carrasco-Valencia, and Serna, 2011; Tang, et al., 2015; Tang et al., 2016).
Quinoa grains are consumed in different forms by the inhabitants of the American Andes: in soups, extruded, instant breakfasts, flour, as a constituent of cookies, flour, bread, tortillas, among others (Bhargava, Shukla, and Ohri, 2006). This is due to their content offiber, starch, and sugars such as maltose, D-galactose and D-ribose, fructose and glucose (Zhu, 2018; Piñuel et al., 2019), which makes them ideal even for the production of fermented beverages such as beer and chicha (FAO, 2011).
The digestibility of quinoa protein is a limiting factor in their use in food (Elsohaimy et al., 2015), but it improves considerably when subjected to germination, fermentation, and thermal treatments, thus increasing the bioavailability of its amino acids (Graf et al., 2014; Navruz-Varli and Sanlier, 2016; Nickel, Spanier, Botelho, Gularte, and Helbig, 2016).
The germination process allows obtaining grains with high biological activity after enzymatic hydrolysis, thus enabling the accumulation of bioactive compounds such as polyphenols (Gan, Wang, Lui, Wu, and Corke, 2016; Televiaute et al., 2020), which improves antioxidant activity (Banchuen, Thammarutwasik, Ooraikul, Wuttijumnong, and Sirivongpaisal, 2009; Sani, Iqbal, Chan, and Ismail, 2012). Thus, the germination process makes quinoa a more functional food (Dávila, Sangronis, and Granito, 2003; Graf et al., 2014; Mariod and Salama, 2020), which implies a new way of sell a nutraceutical food, with good acceptance, especially in people who cannot consume food from animal sources (Piñuel et al., 2019).
The aim of this research was to evaluate the effect of the germination of quinoa (Chenopodium quinoa Willd) of the Salcedo INIA, Pasankalla, and Negra collana varieties on phenolic compounds, antioxidant capacity, and protein content.
Materials and methods
Vegetable material
Organic quinoa grains (grown without the addition of pesticides and synthetic fertilizers) of the Salcedo INIA, Pasankalla, and Negra collana varieties were provided by the Machu Picchu agrarian cooperative in the province of Andahuaylas, Peru. These grains were cultivated during the vegetative period of 2017-2018, in fields located at 13° 39'26" S, 73° 17'32" W, and 3 682 m of altitude.
Obtaining germinated quinoa
Quinoa grains were washed with abundant treated water (pH 7,5), generating friction between grains to eliminate saponin. The washing continued until an evident absence of foam was reached. Then, they were subjected to a humidity between 43% and 45%, and packed a in wet gauze at 35 °C, for periods of 24 and 48 h, in order to cause germination, and the size of the sprout was measured with a Vernier. The sprouts were taken to a horizontal dryer at 60 °C, until constant humidity was reached, and then they were ground to 250 microns in an Agate mortar.
Protein quantification
The nitrogen content was determined through AOAC method 984.13 (AOAC, 2016). The protein content was calculated by N X 6,25, and the results were expressed as percentage on dry basis (d.b).
Total phenol quantification
The spectrophotometric method used byAh-Hen et al. (2012) was followed with some modifications. 1,0 g of sample was taken (germinated quinoa ground) and mixed with 10 ml of acidified methanol with 1% HCl. The homogenate was stored for 24 hours at 4 °C in darkness. Then, it was centrifuged for 15 min at 7000 rpm. An aliquot of the supernatant was taken in a test tube, thus obtaining a germinated quinoa extract, which was stored in a dark refrigerator.
100 μL of the extract were taken and caused to react under agitation with 750 μL of the reagent 1,0 N and 750 μL of sodium carbonate (60 g/L). The mixture was stored for 30 minutes in darkness at room temperature. In the same way, a blank was prepared using distilled water, which served to calibrate the spectrophotometer. Then, the absorbance was read at 755 nm in a spectrophotometer, model T80+ from PG Instruments.
The total phenolic compounds (TP) were estimated from a standard curve made with an aqueous gallic acid solution (R2 = 0,96). The results were expressed as the mg gallic acid equivalent (GAE) per 100 g sample on dry basis (d.b).
Antioxidant capacity quantification
The method of Brand-Williams, Cuvelier, and Berset, (1995) was followed with some modifications. 5,0 g of sample (germinated quinoa ground) were weighed, and 20 ml of 80% methanol were added and mixed for 15 min at 800 rpm. The extract was stored for 24 hours at 4 °C in darkness. Then, it was centrifuged at 3000 rpm for 20 minutes, taking an aliquot of the supernatant (extract) and placed in the darkness at 4 °C lined with aluminum foil.
150 μL of the extract were taken and 2850 μL of diluted DPPH solution (24 mg/100 ml methanol) were added. Identically, a blank was prepared with 150 μL of 80% methanol to obtain a correction factor due to the dilution. The mixture was left to react in darkness for 30 min at 20 °C and then taken to thr spectrophotometer; the absorbance was read at 515 nm.
The antioxidant capacity was calculated using a standard Trolox curve (0,5 mM) and expressed as the iimol Trolox equivalent (TE)/g sample on dry basis (d.b.).
Results and discussion
Sprout size
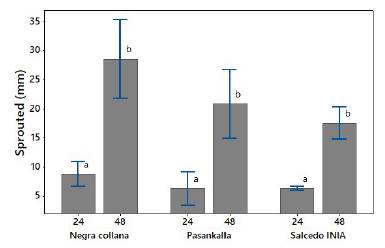
In Figure 1, the size of the sprouts during germination can be observed. After 24 h, sprouts of around 6,4 mm are seen for the Salcedo INIA and Pasankalla varieties, and 8,8 mm for the Negra collana varieties, respectively; after 48 h of germination, these increased significantly (p < 0,05) in 175%, 232%, and 214% for Salcedo INIA, Pasankalla, and Negra collana, respectively (Figure 2). Similar results were reported by Bravo, Reyna, Gómez, and Huapaya (2013) for the Blanca de Junín variety. The difference in sprout size between varieties is due to genotypic differences (Piñuel et al., 2019) and by grain color (Televiaue et al., 2020), as well as composition (sugars, proteins, minerals, lipids, among others) (Han et al., 2019; Piñuel et al., 2019).
Protein content
The protein contents of the ungerminated quinoa grains were between 13,73% and 15,61% (Table 1), with Negra collana being the variety that reported the highest content 15,61 ± 0,23%. These results were similar to those reported by Pereira et al., (2019), and Dakhili etal., (2019).
Table 1 Protein percentage variation in germinated quinoa

Evaluated through a Tukey test at 5% significance.
Source: Authors
On the other hand, it was observed that the protein content in the germinated quinoa increased significantly (p < 0,05) for the Salcedo INIA and Pasankalla varieties, unlike the Negra collana (p > 0,05). Likewise, it was observed that Salcedo INIA reported the greatest increase in protein content, from 13,73% to 14,75% in 24 hours (7,5%), and it increased to 15,18% in 48 hours, which represented 10,6%. On the other hand, Pasankalla and Negra collana reported a 6,1% and 4,8% increase from the initial value after 48 hours of germination, respectively. The same behavior was observed by Chaparro, Pismag, and Elizalde (2010), Bravo et al. (2013), and Piñuel et al. (2019).
During the germination process, the enzymatic systems are activated, mobilizing reserve proteins located in the cotyledons of the quinoa grain. In the same way, changes in the amino acid composition occur due to enzymatic activity (Gan et al., 2016; Televiaute et al., 2020), which allows the production of peptides of intermediate molecular weight due biological activity (Torres, Cova, and Valera et al., 2018; Banchuen et al., 2009). This also happens with the mobilization of nitrogen stored in the quinoa grain, which allows a significant increase in protein, as observed by El-Safy, Mukhtar, and Salem (2013), Graf et al. (2014), and Li, et al. (2014). However, this depends on the humidity and temperature of the environment.
Total phenol content
Polyphenolic compounds are beneficial for human health due to their antioxidant potential, which reduces the risk of cardiovascular diseases, neurodegenerative disorders, and diabetes (Gawlik-Dziki etal., 2013; Navruz-Varli and Sanlier, 2016; Vilcacundo, and Hernandez-Ledesma, 2017; Anngeli et al., 2020).
The TP for the quinoa grains was between 159,69 and 198,23 mg GAE/100 g on dry basis (Table 2). These values are similar to those reported by Abderrahim et al. (2015), Nickel et al. (2016), and Saad-Allah and Youssef (2018). The differences in the total phenol content are due to growing conditions such as soil type (Nsimba, Kikuzaki, and Konishi, 2008; Liberal, Calhelha, Pereira, and Adega, 2016; Huang, Qin, Shi, and Wen, 2017), as well as the variety, since the colored varieties have a higher TP, as reported by Tang et al. (2015), Abderrahim etal. (2015), and Han et al. (2019). Pasankalla (red coloration) reported the highest content, followed by the Negra collana variety (shiny black coloration).
Table 2 TP variation (mg GAE/100 g d.b) in germinated quinoa

Evaluated through a Tukey test at 5% significance.
Source: Authors
It was observed that, after 24 h of germination, the TP for the Salcedo INIA variety had an increase of 61,3% and, 79,0% after 48 h. The Pasankalla variety increased to 55,2% and 110,7% after 24 and 48 h, respectively (Table 2). It could also be observed that Negra collana reported the highest increase (152,6% after 48 h), which is due to its genotypic differences; since this variety is darker, it releases a greater amount of phenolic compounds due to the leaching effect during germination (Televiciute et al., 2020; Piñuel et al., 2019).
The TP increase during the germination process of quinoa has been observed by Alvarez-Jubete, Wijngaard, Arendt, and Gallagher (2010), Filho et al. (2015), Gan et al. (2016), and Piñuel et al. (2019). It is due to the fact that quinoa grains suffer biotic and abiotic stress during germination, which induces the generation mainly of oxygen-reactive spices (ROS). These compounds are vital to protecting the grains during germination (Shulaev, Cortes, Miller, and Mittler, 2008; Televiciute et al., 2020) and for the biochemical and physiological functions of the sprouts, releasing aglycones due to enzymatic activity, which translates into an increase in phenols (Shetty, 2004; Sani etal., 2012). Another critical aspect is the germination temperature, which was 35 °C for this research. The recommended standard is between 35 and 45 °C (Televiciute et al., 2020).
Thus, germination is used as a strategy to increase the presence of bioactive compounds such as phenols, anthocyanins, flavonoids and others in legumes, cereals, and pseudocereals such as quinoa (Mbithi, Van, Rodríguez, and Huyghebaert, 2001; Fernandez-Orozco et al., 2008; Sarvajeet, and Narendra, 2010; Oghbaei, and Prakash, 2017; Televiciute et al., 2020).
Antioxidant capacity (AA)
AA is an aspect that evaluates the ability of a compound to reduce the impact of ROS, and quinoa grains are an excellent source of antioxidants, being superior to many cereals, pseudocereals, and legumes (Pasko et al., 2009; Tang and Tsao, 2017).
In Table 3, it is observed that the ungerminated quinoa grains reported antioxidant activity between 3,18 to 5,65 pmol TE/g d.b. The Pasankalla variety reported the highest antioxidant activity, followed Negra collana. This behavior is common for colored quinoa grains (Tang et al., 2014; Abderrahim et al., 2015; Escribano etal., 2017; Mariod and Salama, 2020). The results were similar to those reported by other authors (Pasko et al., 2009; Tang et al., 2015; Nickel et al., 2016; Repo-Carrasco-Valencia, 2000). However, the variation in antioxidant activity is due to genetic, agrotechnological, and environmental factors (Nsimba et al., 2008).
Table 3 A variation (μmol TE/g d.b.) in germinated quinoa
Evaluated through a Tukey test at 5% significance.
Source: Authors
It was observed that, during germination, the AA of the grains of the quinoa varieties showed a significant difference (p < 0,05). The Salcedo INIA variety reported the greatest increase (19,8% and 69,4% after 24 and 48 h of germination, respectively), while the colored varieties, Pasankalla and Negra collana, reported increases of 20,8 and 19,0% after 48 h, respectively (Table 3). This behavior is common in quinoa grains that germinate under light or darkness (Pasko et al., 2009; Filho et al., 2015; Piñuel et al., 2019); or when subjected to some biotechnological modifications (Nickel et al., 2016; Mariod and Salama, 2020).
The increase in antioxidant activity is due to the response of the seed to the physiological and biochemical changes to which they are subjected at the beginning of germination, so germination is used as a strategy to increase this capacity (Banchuen et al., 2009; Sani et al., 2012; Filho et al., 2015; Torres et al., 2018).
Correlation between variables
It was observed that the size of shoots evinced a high positive correlation with proteins, total phenols, and antioxidant activity (Rs > 0,81) (Table 4), which indicates that germination time causes these variables to increase considerably. Likewise, it was observed that the germination process of the three varieties of quinoa showed a high correlation between the study variables.
Table 4 Correlation of study variables
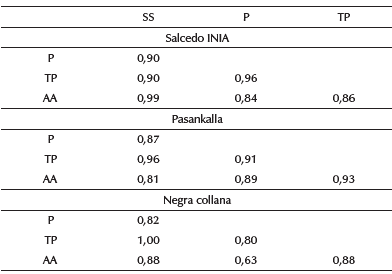
Sprout size (SS), Proteins (P), Total phenols (TP), Antioxidant activity (AA).
Source: Authors
The protein content reported high correlation with the total phenols, with values above 0,80, as well as with the antioxidant capacity. This indicates that quinoa grains, during the stress that occurs during germination, produce phenols and proteins in parallel to condition the sprouts of the grains (Li et al., 2014; Televiciute et al., 2020).
In the same way, the TP shows a high positive correlation with the AA for the three quinoa varieties (> 0,86), which suggests that the phenol content is a good indicator of the antioxidant activity (Pasko et al., 2009; Tang et al., 2014; Contreras-Jimenez et al., 2019), thus making germinated quinoa highly bioaccessible and bioavailable to human health (Tang et al., 2015; Navruz-Varli, and Nevin Sanlier, 2016; Vilcacundo and Hernandez-Ledesma, 2017).
Conclusions
The study showed that unsaponified quinoa grains of the Salcedo INIA, Pasankalla, and Negra collana varieties, subjected to germinations of 24 and 48 hours at 35 °C, increased their content of proteins and total phenolic compounds, as well as their antioxidant activity, presenting a strong positive correlation between them.
The results allow us to confirm that germinated quinoa is a promising product for human nutrition and health.