Introduction
Coal is a heterogeneous mixture of residual plants and associated minerals that have undergone physical and chemical changes that stem from biological and geological processes.
They are chemically composed of oxygen, hydrogen, carbon, and mineral matter, among others. In order to use coal efficiently, it is necessary to implement appropriate cleaning processes to reduce mineral matter. To clean fine coal particles, beneficiation processes focused on the use of flotation columns have been used due to their performance (efficiency) and wide range of operating conditions (Wieslaw, 1994; Polat and Chander, 2003).
One of the most important achievements in fine mineral processing has been the introduction of the flotation column concept in the early 1960s as an alternative solution to the difficulties encountered in mechanical flotation cells. However, it was not until the 1980s that its popularity and commercialization increased, given the growing development of operation and design procedures for mineral beneficiation. The mechanism used by the flotation columns eliminates the entrainment problem (due of the addition of wash water at the top), which is common in conventional flotation machines and generates small bubbles that improve the particle-bubble adhesion process, which contributes to fine particle recovery (Dobby and Finch 1986; Yoon 1993).
Nowadays, flotation columns are highly preferred for the beneficiation of ultra-fine coal. Froth flotation is a solid-solid separation process based on the differences of hydrophobicity between particles. During the flotation process, hydrophobic particles adhere to air bubbles, while hydrophilic particles remaining in the slurry. The successfulness of the mineral matter reduction present in coal is related to the difference in the grade of intrinsic hydrophobicity between the organic matter and the mineral matter present in the coal (Honaker et al., 1996; Polat and Chander, 2003; Pineres and Barraza, 2012).
Some authors determined the effects of frother mixture on froth flotation performance. Three mixed frother systems are designed based on combinations of alcohol, ketone, aldehyde, and polyglycol ether frothing molecules. Frother x is composed of alcohol and ketone, frother y is based on alcohol and aldehyde group chemicals, and frother z is a mixed product of alcohol and polyglycol ether. The results show that frother z is better than x and y in terms of selectivity. In terms of ash reduction and recovery, frother z is effective for coarse and ultrafine particle size fractions. This can be explained by the presence of short chain alcohol molecules (selective for ultrafine fraction) and polyglycol ether molecules (stronger frother for coarse size fraction) in frother z (Gupta et al., 2009).
Peng et al. (2015) studied the effect of flotation reagent adsorption by different fine coal particles on coal flotation. The authors found that low ash fine coal particles have a very strong adsorption to both collector and frother, while high ash fine coal particles have a strong adsorption to collector but weak adsorption to frother, which indicates that frother may play a very important role in the recovery of coarse coal particles (Peng et al., 2015). Other authors studied the effect of methyl cyclohexane methanol and cyclic frothers, as well as comparing coal flotation performance with methyl isobutyl carbinol using two coking coals with different floatability. The results showed that it was an effective alternative to methyl isobutyl carbinol (Hangil et al., 2016).
Without chemical agents (such as collectors and frothers), flotation would not be possible, and, without froth flotation, the mineral processing industry would not have developed as we currently know it. Thus, it is important to understand the effect of the collector and the frother, as well as their role in the performance of the process (recovery and selectivity), especially when Colombian coals are used. The objective of this work was to obtain the highest ash content reduction with the highest mass yield production of two Colombian coal samples using diesel oil (collector) and Flomin F-425 (frother) (information on the use of this frother agent is scarce with regard to cleaning Colombian coals) using a test-rig loop flotation column. One coal sample with high ash content and one with low ash content were selected to evaluate the effectiveness of the reagents (effect) on the selectivity and performance of the process. It should be noted that, theoretically, when a sample has low ash contents, it is more difficult to reduce this value through a cleaning process, because the mineral matter associated with this type of coal (low ash) is normally part of its molecular structure, thus making it very difficult to remove through a physical cleaning process.
Experimentation
Materials and equipment: Two Colombian coal samples, Caypa (North zone) and Guachinte (South western zone), were selected in this research, one with low ash content (Caypa) and another with high ash content (Guachinte) to evaluate the selectivity and performance of the flotation system. The coal particle size used in the flotation column runs was -400 mesh (38 pm), according to the liberation study results. Diesel oil (with a density of 832 kg/m3) and Flomin F-425 (a mixture of alcohol-glycol ether with a density of 1009 kg/m3) were used as collector and frother, respectively.
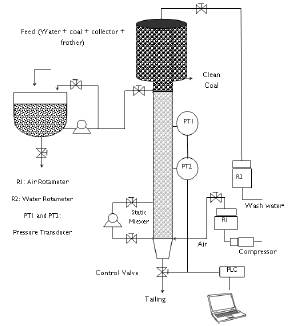
In this project, a 0,05 m diameter, 2 m high flotation column was used. The feed flow rate was 1,25 m, measured from the bottom of the column, while the static mixer for bubble generation and air flow rate was 0,2 m from the bottom of the column. To measure pressure drops, two pressure transmitters were installed along the flotation column (PT1 and PT2). A Danfoss solenoid proportional valve was selected along with a PLC (programmable logic controller). Finally, for communication between control valve and controller, the NB Omron Designer interface was used (Pineres et al., 2019). The test-rig loop flotation column diagram is presented in Figure 1.
In this work, for the contact angle measurements, a goniometer (Rame-Hard) was used using the sessile-drop technique. For the preparation of the polished section specimens, the coal samples were introduced into a hardener solution. Subsequently, the specimens were subjected to a polishing process with sandpaper and, finally, with a solution of aluminum oxide. For each specimen, ten contact angle measurements were made at different sites on the surface of the coal and then averaged. Measurements were made with methylene iodide and double distilled water. These experiments were reproducible within ± 2°. Further description of the experimental development is provided in the referenced literature (Brady and Gauger, 1940; Gutiérrez et al., 1984; Piñeres and Barraza, 2011). For FTIR analysis, Shimadzu IRAffinity-1 equipment was used to account for the identification of functional groups on the coal samples.
Flotation test: Initially, water was added to the preparation tank. Then, the collector was added to the desired concentration, and the specific quantity of coal and frother was added, which produced a slurry concentration of 2,5% w/w used in all experimental tests. This slurry was mixed for 10 min until a homogeneous mixture was obtained. Finally, the feed valve was opened until the desired level height (interface) was obtained inside the flotation column. Table 1 shows a summary of the operating conditions used in the flotation column. The tuning process of the level control loop depends on pressure drop data obtained by the transducers. A set point was selected for the pressure transducers (1,01 psi), in which a froth depth of 58 cm ± 3% and a tail velocity (Jsl) of 0,76 cm/s ± 6% were maintained inside the flotation column in accordance with the design (Bellich, 2016). The float and tailing streams were collected in containers and subsequently filtered, dried, and prepared for the necessary analyses.
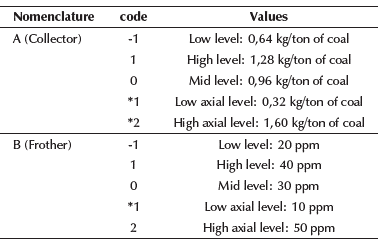
Experimental design: In this research, a response surface methodology was used (Hicks, 1982; Montgomery, 2013), where the effect of diesel oil (collector) and a mixture of alcohol-glycol ether (frother) on mass yield and ash content of two coal samples was evaluated. Table 2 shows the coding used during the experimental development (A: collector concentration, B: frother concentration). Collector concentration was selected because of its influence on coal hydrophobicity, while frother concentration was selected because of its influence on bubble diameter.
The experimental error was 5%. Initially, experimental tests that make up factorial design and its center points were performed. Later, the correlation between the response variables and the input variables was determined using a second-order model. If the quadratic terms (curvature) were not significant, the experimental test stopped and the data were fixed to a first-order correlation. Otherwise, it was necessary to perform the axial points and propose a new model (Hicks, 1982; Montgomery, 2013). The ash analysis was performed in duplicate, and the deviations from the average value of the response were a mass yield of 5% and an ash content of 0,5%.
Results and discussion
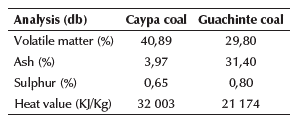
Coals:Tables 3 and 4 show the proximate analysis and contact angle values of raw coal.
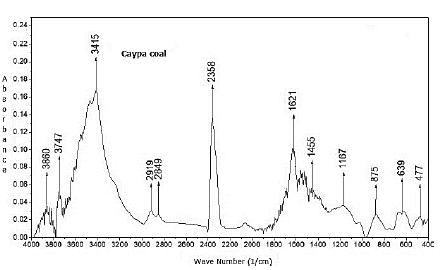
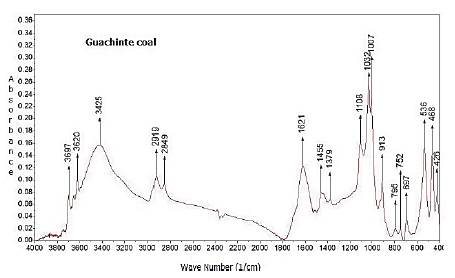
The results indicate that Caypa coal and Guachinte coal are bituminous (Leonard and Hardinge, 1991). Caypa coal exhibits higher values in volatile matter than Guachinte coal. It was observed that the ash content for Guachinte coal (31,4%) exhibits a higher value than Caypa coal (3,97%). Caypa coal and Guachinte coal had relatively close sulfur contents (0,65 and 0,8%, respectively). Ash content, heat value, and contact angle are correlated with each other, as can be seen in Tables 3 and 4, where Caypa coal has a low ash content (3,97%) with a higher heat value (32003 KJ/kg) and contact angle (63,1 °), compared to Guachinte coal, which has higher ash content (31.40%), with low heat value (21174 KJ/kg) and contact angle (60,9°). These results may be attributed to the difference in hydrophobic sites on the coal surface of each sample, indicating that the Caypa coal could have a higher degree of hydrophobicity than Guachinte coal. This result may be due to the chemical (quantity and types of functional groups on the surface of the coal) and geological nature of each sample. The contact angle values in methylene iodide are lower than those measured with water (Table 4), which could be due to the increase in the dispersion component on coal surface. (Gutiérrez et al., 1984; Piñeres and Barraza, 2011,2012). Figures 2 and 3, which do not have the same scale, show the spectra obtained using Fourier Transform Infrared Spectroscopy (FTIR) on both coal samples, with which it was possible to qualitatively identify the functional chemical groups present. Qualitatively, the intensity of each peak at a given wavelength is considered to be directly related to the group concentration. The spectrum shows a vibration variety corresponding to groups such as: -NH-NH2-OH- (3 400-3 700 cm-1), CH2, CH3 (2 850-2 920 cm-1), -C=O-O-CO, C=C-OH (aromatic), C=O, C=C, aromatic -OH (1 580-1 800 cm-1), minerals (460-540 and 1 000-1 050 cm-1), aromatic (600-920 cm-1), C=O, aromatic-O-aromatic (1 100-1 380 cm-1), nitrite groups attached to alkenes and aromatic structures (1 330-1 530 cm-1), short aliphatic chains (2850 cm-1), and long aliphatic chains (2920 and 3920 cm-1).
It is worth highlighting that these bands represent characteristic groups, and a specific structure cannot be specified, which may be attributed to the complex chemical structure of coal. For example, the C=C group can be attached to simple or complex aromatic structures. The characteristic wavelengths of these groups were taken from other works carried out with bituminous coals (Sobkowiak et al., 1984; Cooke et al., 1986; Solomon and Carangelo, 1988; Shu et al., 2002). Based on the reported wavelengths, Figure 3 shows that Guachinte coal presents a large number of functional groups that contain oxygen, -C=O-O-CO, C=C-OH (aromatic), C=O, C=C, and aromatic-(OH) in comparison with Caypa coal (Figure 2). Caypa coal has less minerals than Guachinte coal, which can be corroborated through proximate analysis (Table 3), with Guachinte coal having the highest mineral quantity (Table 3) and the highest intensity of the corresponding band. Caypa coal has a lower concentration of the aromatic-O-aromatic group compared with Guachinte coal. It was also observed that the two coal samples show close values regarding the CH2, CH3 and -NH-NH2-OH- groups.
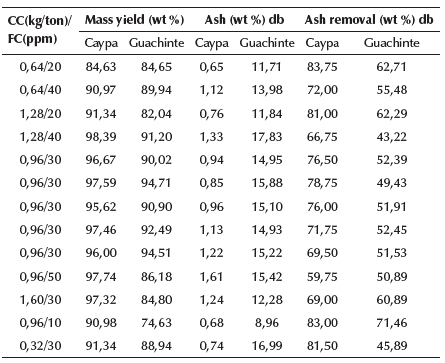
Effect of Flomin F-425 and diesel oil on mass yield and ash percentage of the clean coal: The effect of collector and frother concentration on mass yield, ash content, and ash removal of floats on a dry basis is shown in Table 5. It was observed that Caypa coal reported a yield higher than 80% (84,63-98,39%). The highest value was obtained by adding 1,28 kg/ton of coal and 40 ppm of collector and frother concentration, respectively; while, for the Guachinte coal, a yield range between 74,63 and 94,71% was obtained. Its highest value (94,71%) was reached at 0,96 kg/ton of coal of collector concentration and 30 ppm frother concentration. It is also shown in Table 5 that, for all the conditions used in the experimental procedure, the coal samples generally showed a significant decrease in the ash content with respect to their initial value (3,97 and 31,4%, Table 3). The largest value in the ash removal for Caypa coal was 83,75% (0,65% of ash content), obtained at a collector and frother concentration of 0,64 kg/ton of coal and 20 ppm, respectively; whereas, for Guachinte coal, the greatest ash removal value was 71,46% (8,96% of ash content), which was reached at 0,96 kg/ton of coal of collector concentration and 10 ppm of frother concentration.
It is worth highlighting that the obtained ash content of 0,65% is the lowest value reported in the literature using a single-stage test-rig loop flotation column (without chemical reagent addition such as HF and HCl, among others), which is considered to be an ultra-clean coal obtained through a physical cleaning process. The sample had an initial ash content of 3,97%, which is considered relatively low. Theoretically, the lower the ash content in a coal sample, the greater the degree of difficulty in reducing this value through a physical cleaning process, because the mineral matter associated with this coal type (low in ash) is normally part of its molecular structure, thus making it very difficult to eliminate. This indicates that the coal sample used had a high degree of mineral matter release. Likewise, the flotation column had a bubble size distribution that promoted a good bubble-coal particle adhesion, thus proving the effectiveness and selectivity of test-rig loop flotation columns (Piñeres and Barraza, 2012).
The results show that the mass yield tends to increase with the frother concentration increase for the two coal samples (Table 5). The maximum mass yield values for Caypa coal (98,39%) and for Guachinte coal (94,71%) were obtained by adding 40 and 30 ppm of frother concentration, respectively; whereas, for ash removal, an opposite effect was observed: with the frother concentration increase, the ash removal tends to decrease (the ash content increases when the frother concentration increases) for both samples. The maximum value of the ash removal for Caypa coal was 83,75% (the lowest value for ash content was 0,65%), and, for Guachinte coal, it was 71,46% (ash content of 8,96%). They were obtained when 20 and 10 ppm of frother concentration were added, respectively.
The high mass yield obtained at high frother concentrations is due to the decrease in bubble diameter obtained under these conditions (increase in the frother concentration) as a consequence of water surface tension decrease (Fuerstenau and Pradip, 1982; Finch et al., 2008; Piñeres and Barraza, 2012). This effect may be attributed to mass transfer at the water-frother interface, which produces a surface tension gradient, thus generating spherical, small-sized air bubbles with a rigid surface (increase in available surface area of air bubbles) and, likewise, increasing air bubble hydrophobicity, favoring particle-bubble adhesion and increasing the mass yield. According to the results in Table 5 we can see that the test-rig loop flotation column used reported a good performance for coal beneficiation (Fuerstenau and Pradip, 1982; Finch etal., 2008).
The frother used was Flomin F-425, a non-ionic, completely water miscible reagent that has a hydrophilic polar group (mixture of alcohol) at one end and a hydrophobic non-polar group (glycol ether) at the other. Its molecular structure can be represented as: CH3-[-O-C3H6]n-OH. Due to its amphiphilic nature, Flomin F-425 orients its hydrophilic group (polar) towards water, while its hydrophobic group (non-polar) is oriented towards the air bubble. The action of Flomin F-425 at the air bubble-water interface seems to be halfway between being completely lying down and fully standing, which suggests the formation of loops and coiling up at the interface with the frother molecule. The addition of Flomin F-425 to the slurry (coal-water) breaks the hydrogen bridges that form the water molecule (the hydrogen bridges create high surface tension in the water) and decreases its surface tension, causing the molecules to tend to lie at the surface, possibly increasing the viscosity of the froth and, therefore, their stability. The action of this frother can be attributed to the interaction of the Flomin F-425 oxygenated polar group (Flomin F-425 has several oxygenated groups in the molecular chain), which acts strongly with the oxygen-containing functional groups on the surface of the coal through hydrogen bridges (decreasing hydrophilic sites on coal surface), thus generating a stable coal particle-bubble adhesion (Fuerstenau and Pradip, 1982; Finch et al., 2008; Gupta et al., 2009).
Table 5 shows that the mass yield tends to increase lightly with the collector concentration increase for Caypa coal, the maximum mass yield value (98,39%) was reached when 1,28 kg of collector/ton of coal was added. On the other hand, the opposite effect occurred with ash removal; when the collector concentration increases, ash removal tends to decrease slightly (ash contents increase with the collector concentration increases). The maximum value of the ash removal for Caypa coal was 83,75% (ash content of 0,65%), which was obtained at 0,64 kg of collector/ton of coal. Similarly, for Guachinte coal, the mass yield tends to decrease when the collector concentration increases. The maximum value obtained for mass yield (94,71%) was reached when 0,96 kg of collector/ton of coal were added. The opposite effect occurred once more with ash removal; with the increase in collector concentration, ash removal tends to increase slightly (ash contents decrease when the collector concentration increases). The maximum ash removal value for Guachinte coal was 71,46% (ash content of 8,96%), which was obtained at 0,96 kg of collector/ton of coal.
Diesel oil, a non-polar type of hydrocarbon (insoluble in water), was used as collector. It is approximately 75% aliphatic and 25% aromatic with the following molecular structure: Ci2H26. It adheres to the coal surface in order to increase hydrophobicity and improve particle-bubble adhesion. The action mechanism of diesel oil may be due to the interaction of the non-polar chain with the carbonaceous (hydrophobic) sites on the surface, displacing the water molecules and facilitating their adhesion. The action of this collector can be attributed to hydrophobic bonding of the aliphatic chain and n bonding of the aromatic rings with the hydrophobic and aromatic sites on the coal surface (Jia et al., 2002).
The differences in the behavior of the two coal samples with respect to the employed frother and collector concentrations mainly depend on the mineral types, occurrence mode, and ion concentration (valence) of the coal structure. Nevertheless, the liberation grade of the mineral matter during the grinding process and the interaction between flotation reagents (collector and frother) and coal surface affects floatability and the selectivity of the test-rig loop flotation column (Fuerstenau et al., 1983; Arnold and Aplan, 1986a,1986b).
Statistical analysis: The data for mass yield and ash content were statistically analyzed using the Design Expert software. Table 2 presents the nomenclature used, and Table 6 shows the results of the statistical analysis of the variables and their interactions. The regression models for each coal are described using Equations (1) to (4):
Caypa coal:
Guachinte coal:
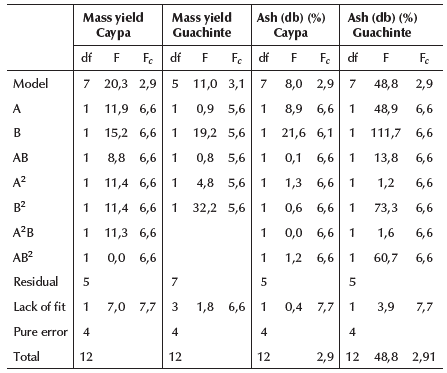
For both studied coals, Table 6 shows that these model equations have a significant effect (F > Fc), whereas the lack of fit shows no significant effect (F < Fc), thus indicating that the expression found is a reasonable approximation for the surface in the region considered in the experiment (Hicks, 1982; Montgomery, 2013).
Table 6 shows that collector and frother concentrations present a significant effect on mass yield for Caypa coal (F > Fc), while, for Guachinte coal only frother concentration presents a significant effect (F > Fc). Meanwhile, collector concentration does not report any significant effect (F < Fc). Likewise, curvature effects were found for the different variables used, showing that, in the case of mass yield, the collector and frother concentrations are significant for the quadratic terms (A2, B2) on Caypa coal (F > Fc), while Guach-inte coal only reports a significant effect in the quadratic term of the frother concentration (B2) (F > Fc). It is also possible to notice that the interaction between AB and A2B is only significant (F > Fc) for Caypa coal (Hicks, 1982; Montgomery, 2013).
The significance of the frother concentration on the Caypaand Guachinte coals indicates an effective interaction between frother agent and coal surface, which increased floatability. Nevertheless, bubble hydrophobicity can be increased as a consequence of the association of water molecules with the frother's non-polar group on the surface of the bubbles, thus promoting coal particle-bubble adhesion. The significance of collector concentration (F > Fc) on the Caypa coal shows the interaction between the collector and the coal's surface. The opposite effect was observed for the Guachinte coal, where collector concentration was not significant (F < Fc). This may be attributed to the lack of affinity between the collector used and coal surface, due to the differences in their chemical and mineralogical structures (mineral specie and valence), surface properties, and the functional groups of the samples. Therefore, the collector agent shows a different behavior for each coal sample (Jia et al., 2002).
Table 6 shows that collector and frother concentrations have a significant effect on ash content for the two coal samples (F > Fc), while the frother concentration of Guachinte coal (F > Fc) has a significant curvature effect (B2, AB2). It is also possible to notice that the interaction AB and AB2 is significant (F >Fc) only for Guachinte coal, which shows that the flotation reagents (collector and frother) interact with mineral matter on the surface of the coal, thus affecting the selectivity of the flotation process (Hicks, 1982; Montgomery, 2013).
The differences in the values of the mass yield and ash content may be attributed to the differences in the hydrophobicity of each coal, as a consequence of the different degrees of carbonization, petrographic composition, mineralogical distribution, and chemical nature.
Conclusions
Caypa and Guachinte coals presented high mass yield values and low ash content values for all flotation column operation conditions. Caypa coal reporded a yield higher than 80%, (84,63-98,39%), and the highest value was reached by adding 1,28 kg/ton of coal and 40 ppm of collector and frother concentration, respectively; while Guachinte coal obtained a yield range between 74,63 and 94,71%. Its highest value was obtained at 0,96 kg/ton of coal of collector concentration and 30 ppm frother concentration. The lowest ash content value for Caypa coal was 0,65%, obtained at collector and frother concentrations of 0,64 kg/ton of coal and 20 ppm, respectively; whereas, for Guachinte coal, the lowest ash content value of ash content was 8,96%, obtained at 0,96 kg/ton of coal of collector concentration and 10 ppm of frother concentration. For Caypa coal, the mass yield tends to increase (low ash removal) when the collector and frother concentrations increase, while the mass yield of Guachinte coal tends to decrease (high ash removal) when the collector concentration increases (low ash removal) at high frother concentrations. The ash content of 0,65% obtained is the lowest value reported in the literature using a single-stage test-rig loop flotation column.