Introduction
Coffee trees are shrubs that produce fleshy fruits called coffee cherries, which are to date considered to be the most popular natural products. The particularity of their structure allows a glimpse into two well-known species in the coffee sector, which, when processed separately, have different organoleptic properties. Industrialization has allowed for a synergy between them, aiming to achieve the characteristic flavor and aroma of coffee (Andrade and Zapata, 2019).
Nowadays, two predominant varieties take up the whole of commercialization. The first one is called Arabica, whose peculiarity is its extended flattened shape, with ovals and a thin dividing line in the shape of an "S". In addition, its properties diffuse a striking aroma, ideal in taste and quality. However, its caffeine percentage is half when compared to its rival. The second variety is Robusta, which is characterized by a spherical structure and a slightly straight median line. Its flavor is intense and bitter, and it has greater consistency, tentatively reducing its quality, but it has more caffeine than Arabica (Venegas et al., 2018).
Both varieties are detailed in Figure 1, emphasizing their physical structure so they are easily distinguished.

Figure 1 Structure of the varieties of roasted coffee: a) Arabica species (cultivation altitude between 900 and 2 000 m at temperatures of 15-25 °C), b) Robusta species (cultivation altitude between 0 and 900 m at temperatures of 20-30 °C.
Currently, coffee beans are used in the preparation of drinks for frequent consumption, given their pleasant taste and smell, as well as the stimulating properties of caffeine (Carvajal, 2019). Thus, in response to the high demand for coffee, the industry is constantly growing, increasing the amount of waste generated, which reaches figures of around 6 million tons of remainder (Carvajal, 2019). Pulp, mucilage, parchment, and lint are discarded as by-products of the process. These residues are known as Spent Coffee Grounds (SCG), and they do not have commercial value, so they are disposed of through an adjusted treatment, since their content of tannins, polyphenols, and caffeine has a negative impact on the environment (Silva et al., 1998). However, reusing these residues has been proposed for the evaluation of phenolic compounds that represent commercial interest (Magalhães et al., 2016).
In light of the above, a coffee industry was selected in Guayaquil, Ecuador, which involved the industrialization of soluble coffee, since a large amount of waste is generated from coffee grounds during its processing, which constitutes an alternative source to obtain phenolic compounds. It is necessary to underline that, given the astringency of coffee, some studies have recommended the possibility of extracting phenolic compounds such as tannins. In this context, to shed light on the issues, our exploration was carried out in an instant coffee factory dedicated to manufacturing two commercial types of coffee: freeze dried (granulated) and spray dried (powder).
The method used to extract phenolic compounds plays an important role in the quality of the extract. There are various types of extraction, e.g., (i) microwave-assisted extraction, (ii) ultrasonic extraction, (iii) accelerated solvent extraction, (iv) supercritical extraction, and (v) ultrasound-assisted techniques, whose extraction results are based on the phenomenon of acoustic cavitation (Soto-García and RosalesCastro, 2016). Ultrasound induces a series of physical, chemical, and mechanical effects or impacts, causing an increased recovery rate of phenolic compounds (Rodríguez, 2020). In this study, continuous solvent extraction through Soxhlet leaching was implemented, generating the displacement of soluble substances contained in a solid of interest through mass transfer, which was caused by a highly selective solvent that avoids chemical transformations (Gutiérrez, 2017).
Ultimately, the purpose of this study is to detect the influence of the solvent during the extraction of coffee grounds and justify the best option based on its dielectric constant, the degree of intervention during the process, the cost, and the presence of unwanted reactions. Moreover, the quantitative presence of phenolic compounds is determined via UV-VIS spectrophotometry characterization and qualitative staining/ precipitation tests.
Methodology
Regardless of the two varieties of the coffee bean, the harvested ripe fruit goes through a transformation process to become the so-called green coffee, aiming to strip the grain of the multilayers, in the following order: husk, pulp, mucilage, and parchment. Subsequently, the fruit goes through the roasting process, and the physicochemical characteristics of the accepted grain are evaluated for reception in storage silos, where they are transported to the continuous roaster to acquire their organoleptic properties. The roasting must be uniform, with a residence time of 8-12 minutes at a temperature between 240 and 260 °C, so that the output grain can reduce its humidity, obtain its characteristic structure, and vary its chemical composition (Torres-Valenzuela et al., 2019).
From there, the grain goes through a milling stage, where it reaches the required granulometry (300-600 pm). During solid-liquid extraction, the components of the bean, called coffee extract, are absorbed into the water. The accumulation of the residues is what is known as coffee grounds. Finally, the coffee extract must be centrifuged to enter the final dryer, which can be done by freeze-drying or atomization (Calle and Mendoza, 2017).
In Table 1, the amounts of the by-products of the process are detailed from 1 000 g of coffee cherry.
Table 1 By-products of coffee cherry processing
| Process stage | By-product | Loss (g) |
| Pulp removal | Pulp | 394 |
| Fermentation | Mucilage | 216 |
| Drying | Water | 171 |
| Threshing | Parchment | 35 |
| Roasting | Volatile compounds | 22 |
| Beverage preparation | Coffee grounds | 104 |
| Total Loss | 942 |
Note: Calculation base of 1 000 g of cherry coffee with 5,8% utilized for beverage preparation
Source: Authors
Coffee grounds are the product of solid-liquid extraction, but some studies have shown that they contain 10-15% of oil on a dry basis (Calle and Mendoza, 2017). Likewise, it has been discovered that only 1% of oil is transmitted to the coffee extract, which makes it possible to work with this by-product in order to obtain phenolic compounds.
Materials and equipment
Coffee grounds (mixture of robust and arabica species in a ratio of 9:1) were obtained from a coffee industry in the city of Guayaquil, Ecuador. All reagents used were of analytical grade: lead acetate Pb(C2H3O2)2 reagent, gelatinsalt reagent (1% gelatin and 10% sodium chloride solution), ferric chloride (FeCl3) reagent, bromine water, and distilled water. The following lab equipment was available: Soxhlet XL equipment, a Rotavapor R-1 020, a 3 840-L centrifuge, and an electric grain mill (HCP).
Sample preparation
For ten weeks, with a periodicity of three days a week, 90 kg of wet coffee grounds were collected. Then, outdoor drying was carried out on concrete surfaces with a slope of 1% in order to avoid entrapment, and, in accordance with the INEN-ISO 712:2013 standard, tests were carried out in a stove until a relative humidity of 5% was reached. In the next step, the particle size was reduced using an electric grain mill, until a granulometry between 250 and 500 um was obtained.
Solvent selection
The polarization of a substance can be determined, as well as its ability to associate with other molecules through the relationship with its dielectric constant. For this reason, at the atomic scale, molecular polarization can be affirmed when the separation between the centers of gravity of the positive and negative charges is evidenced. For water, this condition is permanent. However, the conditions of other solvents can be predicted according to the molecular geometry of each compound. In other words, for materials or compounds formed by molecules with constant polarity, there is a common characteristic: high dielectric constants (Guadarrama, 2020).
In accordance with the above, and highlighting the use of solvents, Table 2 shows satisfactory characteristics according to the type of bond, the molecular geometry, and the dielectric constant.
Table 2 Solvent characteristics
| Solvent | Polarization (bond | Molecular | Dielectric |
|---|---|---|---|
| type) | geometry | constant | |
| Water (H2O) | Polar covalent (hydrogen bonds) | Angular | 78,5 |
| Methanol (CH3OH) | Polar covalent (dipole-dipole force) | Tetrahedral | 33,0 |
| Ethanol | Polar covalent | Flat | 24,0 |
| (C2H5OH) | (dipole-dipole force) | Triangular | |
| Hexane (C.HJ | Nonpolar covalent (induced dipole force) | Tetrahedral | 2,0 |
Source: Authors
In order to thoroughly examine the factors that influence the selection of the solvent, this study provides an analysis of its intervention in the process and the possible unwanted reactions that may constitute significant hazards.
Soxhlet leaching
366 g of dry and ground residue material (coffee grounds) were selected as a sample for extraction with solvents of increasing polarity. The performance of three different solvents was evaluated: 1 000 mL of 96% Ethanol, 95% Methanol, and 97% Hexane, maintaining temperatures of 80, 67, and 70 °C respectively, in an interval of 120 minutes. Then, for each test, a mixture of solvent with oil extracted from the coffee grounds was obtained and filtered when cold. Later, the solvent was separated using the Rotavapor, and a portion of extracted oil was obtained (Torres, 2020), which was stored for subsequent qualitative and quantitative analysis (López-Bascón and Luque de Castro, 2020).
Quantitative determination of phenolic compounds
The content of phenolic compounds was determined via the Folin-Ciocalteu method, as described by Muñoz-Bernal et al. (2017), with some variations. 240 u± of extract were combined with 15 mL of deionized water and 3 mL of the Folin-Ciocalteu (2N) reagent for phenols. After 5 min, 3,8 mL of Na,CO3 (8%) and 25 mL of deionized water were added to the mixture. The absorbance was quantified at 760 nm in a UV-VIS spectrophotometer (Mettler Toledo UV7). The content was calculated from a standard of gallic acid in mg (gallic acid equivalent, GAE) per 100 mg/L, with a regression equation and a gallic acid calibration curve. All experimental conditions and analyses were replicated three times for each extract.
Qualitative determination of phenolic compounds
In the search for phenolic compounds, this research focused on the determination of tannins, due to their high degree of interest within the industry under study. To this effect, the coffee grounds oil obtained via Soxhlet leaching was filtered and deposited in four test tubes, each one with 1 mL aliquots, in order to perform the analysis with precipitation reagents and phenolic compounds staining to identify and differentiate the tannin classification obtained during experimentation.
In the first test tube, the presence of hydrolysable compounds was investigated from a 10% solution of Pb(C2H3O2)2. If there is such presence, the precipitate should be white due to the lead salts linked to phenolic compounds. In the second tube, a gelatin-salt solution scanned for the presence of hydrolysable compounds by means of a white precipitate. The third tube used 10% FeCl3 to detect two types of ferric salts: (i) green staining: non-hydrolysable or condensed compounds; and (ii) blue staining: hydrolysable compounds derived from pyrogallol. Finally, the fourth tube used bromine water for the detection of condensed phenolic compounds (Colina, 2016). The tests were carried out in triplicate for each solvent.
Lead acetate test
1 mL of 10% Pb(C2H3O2)2 was added to 1 mL of the ethanolic and methanolic extracts. Given the presence of a precipitate, the mixture must be isolated, and 5 drops of a dilution of acetic acid (CH3COOH) must be added. All natural tannins precipitate with lead acetate in neutral solutions; the filtrate does not react with a salt that includes iron. The precipitate of flobatannins (cathekic tannins) is dissolved in diluted CH3COOH. Finally, the gallotannins precipitate is partially or totally insoluble.
Gelatin-salt test
Three drops of gelatin-salt were added to an extract of 1 mL ethanol and 1 mL methanol. Initially, a compound in the form of a cloud appeared in the solution. Later, the mixture was placed in a centrifuge. After this process, a white precipitate was observed, which indicated the presence of tannins.
Ferric chloride test
Two drops of FeCl3 were added to both, a 1 mL methanolic extract and another 1 mL ethanolic extract. With this test, if it was possible to observe a bluish-black coloration, the presence of tannins in the pyrogallol derivatives was confirmed. On the other hand, if the coloration was green, it meant that the tannins came from catechin.
Bromine water test
Five drops of bromine water were added to two extracts: 1 mL of methanol and 1 mL of ethanol. In this test, it was possible to verify the presence of a cathekic tannin if a precipitate formed.
Results and discussion
Extraction performance
For the extraction of the coffee grounds oil, three different solvents with increasing polarity were used: hexane, ethanol, and methanol. This, in order to determine the highest extraction yield for a 120-minute interval according to the solvents' boiling point. Thus, the first extraction method, which involved hexane, recorded a low yield close to 7% and reported the presence of nonpolar secondary metabolites (Mena-Valdés et al., 2015). In the second extraction (ethanol) the presence of polar-related compounds was evidenced, achieving almost 3,3 times the yields of hexane. Finally, methanol extraction surpassed the previous values. Table 3 shows the values achieved by each extract based on the final residence time for the extraction of waste.
Table 3 Solvent extraction performance
| Solvent | Yield | Residence time |
|---|---|---|
| Hexane | 7,2% ±0,11a | 120 min |
| Ethanol | 23,8% ±0,27b | 120 min |
| Methanol | 27,1% ±0,27b | 120 min |
Note: Evaluated with a Tukey statistical test with 5% significance
Source: Authors
Finally, based on the results, the yield obtained with the methanolic extract was the highest. Most of the substances or components present in the coffee grounds show an affinity with the methanol compound. However, due to the methanolysis of gallic depsides, this compound is not recommended (Marcelo-Díaz et al., 2017). It is also worth noting that the yield percentage measures the number of compounds related to the polar nature of the solvents used for extraction.
Quantitative analysis: UV-VIS spectrophotometry
To compare the effect of the solvents, the influence of their dielectric constant and the type of bond can be evidenced. Average phenol values were obtained from all Soxhlet extraction values. The methanolic extracts showed higher content of polar compounds, while the ethanolic ones had a higher amount of phenolic compounds. In addition, the extracts with hexane were rich in highly unstable, polyunsaturated fatty acids (Corona-Jiménez et al., 2016). The results for the total phenol content of the solvents showed significant differences when comparing the extraction times (90 and 120 minutes vs. 60 minutes). In the samples with hexane, there were extraction difficulties, which is explained by its low affinity to hydrophilic compounds, given their nonpolar covalent character. Similarly, there is an influence of the polarity of the solvent on the extraction process. Thus, the highest phenolic compounds were obtained in samples with ethanol. This value decreased during the extraction with methanol, and it remained constant in hexane, with lower values in comparison with the other two solvents.
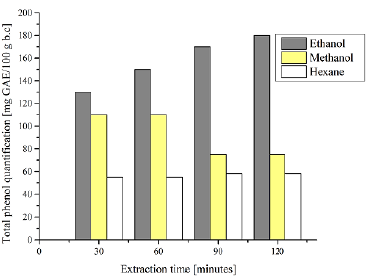
Source: Authors
Figure 2 Effect of the solvents during four periods of extraction of phenolic compounds from coffee grounds by means of the Soxhlet method
The results show a significant difference between the phenolic compounds of the ethanolic and methanolic extracts for different extraction periods. These compounds, which are present in coffee grounds, are characterized by their polar nature. Similarly, the liquid-solid ratio was set for the three types of solvent extraction, and the optimal conditions were an extraction time of 90 min and a 96% ethanol concentration. In comparison with other publications (Marcelo-Díaz et al., 2017), ethanol turned out to be an optimal alternative for the extraction of phenolic compounds, which is due to its polar affinity. Moreover, when it is assisted by ultrasound, an increase in extraction yields can be achieved. Table 4 shows the univariate analysis of variance conducted in order to confirm the influence of the solvent on the Soxhlet-assisted extraction.
Table 4 Univariate analysis of variance
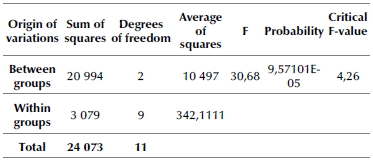
Note: One-way ANOVA for three extraction solvents
Source: Authors
The groups in the Table are made up of the three solvents: ethanol, methanol, and hexane. The contrasted hypothesis for the study from the unifactorial ANOVA (H0) and establishes that there are no significant differences between the solvents. However, the results indicate a probability (P) lower than 0,05, which allows rejecting the null hypothesis. Moreover, since F is greater than the critical F-value, the results obtained from the tests are significant. Similarly, to give greater clarity regarding the choice of the optimal solvent, Table 5 complements the results of the ANOVA with a statistical analysis assisted by the Tukey test.
Table 5 Statistical Tukey analysis
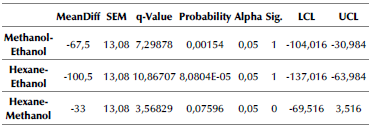
Note: If Sig. is equal to 0, the difference of the means is not significant at the 0,05 level. If Sig. is equal to 1, the difference of the means is significant at the 0,05 level.
Source: Authors
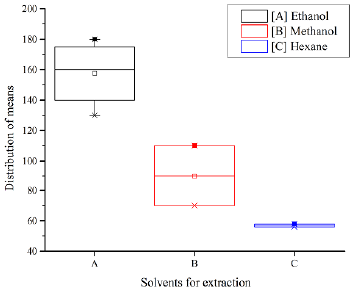
By comparing the treatments used, it is observed that the difference between means the corresponding to Methanol-Ethanol and Hexane-Ethanol are large. Therefore, with p<0,05, both pairs represent different treatments, which is why ethanol is considered to be the optimal solvent, as it differs significantly with respect to the value of its means. Similarly, Figure 3 shows the data distribution for each solvent extract and the means obtained in triplicate with each extraction method.
Qualitative analysis: coloration and precipitation
For the qualitative identification of the phenolic compounds called tannins, this study was framed in the use of ethanolic and methanolic extracts, discarding the extract with hexane because of its nonpolar affinity. Consequently, given the varieties of phenolic compounds, the study focuses on hydrolysable tannins, as they undergo hydrolysis in the presence of acids, enzymes, and basic media in a polyol or sugar added to the phenolcarboxylic acid. Subcategories are established in relation to the acid used: gallotannins (gallic acid) and ellagitannins (ellagic acid).
Table 6 Qualitative determination of phenolic compounds (tannins)
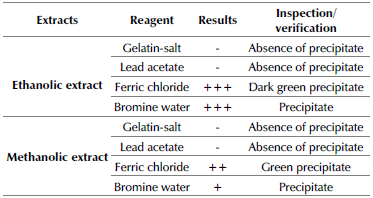
( + ) Scarce presence, (++) moderate prescence, ( + + + ) abundant presence, (-) not detected
Source: Authors
When precipitated in bromine solutions and treated with acids at high temperatures, the condensed tannins from catechol generate a gradual green polymerization. In this way, the analyses indicate the presence of phenolic compounds such as tannins in coffee grounds, both for ethanolic and methanolic extracts. However, it is clear that there is a greater amount of total phenols in ethanol, which makes it possible to discard the use of methanol as an extraction solvent in this context.
Conclusions
The criterion for selecting the optimal solvent involved a high selectivity or affinity towards polar compounds. Therefore, based on the relationship with its dielectric constant, water is considered an ideal solvent, since soluble compounds (hydrolysable tannins derived from pyrogallol) and their ability to associate with other molecules have been extracted in the different stages of coffee processing. Although the use of methanol has a higher dielectric constant and extraction efficiency, it can cause of the formation of toxic substances and the methanolysis of gallic depsides. Hexane is not favorable either, given that if the dielectric molecules have a temporary dipole moment (nonpolar molecules), their association capacity tends to decrease due to their dielectric constant. In addition, hexane is considered one of the toxic alkanes due to the physiological effect caused by the products of its metabolization. One of them is 2,5-hexadione, which reacts with essential amines and is a neurotoxic agent. Thereupon, ethanol was established as the optimal solvent in this study, in view of its ease of handling, lower toxicity, polar covalent character, relevant dielectric constant, absence of unwanted reactions, and increased yield of phenolic compounds during extraction.
To date, a wide variety of procedures have been used for the purpose of extracting phenolic compounds, but most of them begin with extraction by organic solvents or with some mixture involving them. In particular cases, it is possible to combine and calculate the capacity of a mixture of solvents to induce a dipole moment, since the dielectric constant of the system depends on the constants of each of the substances and their percentage in the mixture. However, harmful effects may be caused by their synergy. Complementary alternatives are needed to improve solventassisted extraction on an industrial scale, so as to increase the yield and quality of the extracts. There is a constant search for the correct process variables, as the use of this technology is subject to industrial objectives and operating conditions.
Finally, the solvent-assisted extraction of phenolic compounds constitutes a viable and innovative alternative use of coffee grounds. The results of the one-way ANOVA indicated the influence of the solvent in the extraction process. The null hypothesis was rejected for two reasons: a sufficiently low probability and a very large variability relationship, where F was greater than the critical F-value. In addition, the Tukey test and the box-and-whiskers diagram allowed selecting ethanol as the optimal solvent for the leaching of coffee grounds, given the increased yield of phenolic compounds obtained with a residence time of 120 minutes, which shows promise for subsequent tannin applications in parallel industries.