Introduction
The three main pathological stages in coronary artery disease (CAD) are the process in pre-intima, in-intima, and post-intima1. Of these, the pathological mechanism in post-intima —defined as platelet activation, secretion, and aggregation— is the most responsible to cause clinically acute myocardial infarction2,3. This process involves several proteins including von Willebrand factor (vwf)4, thromboxane A25, collagen6, fibrinogen7, and vesicle-associated membrane protein-8 (VAMP-8)8. Of these, studies concerning the correlation between VAMP-8 and acute myocardial infarction had a limited number. Whereas, elevated levels of VAMP-8 had been shown to be correlated with platelet hyperreactivity and overexpression9, platelet granule secretion8,10,11, and thrombus formation11. Moreover, by evaluating the levels of VAMP-8 messenger ribonucleic acid (mRNA), a study found that VAMP-8 mRNA levels were higher in hyperreactive platelets12.
Recently, several single-nucleotide polymorphisms (SNPs) of VAMP-8 gene have been reported, such as rs1010, rs1058588, rs1009, rs13421434, rs1348818, rs3731828, rs7579147, rs3770098, and rs675726313,14. Until now, it is unclear which SNP plays a crucial role in determining the VAMP-8 levels in the circulation. Interestingly, in the SNP database (https://www.ncbi.nlm.nih.gov/snp/?term=vamp8), it was showed that rs1010 (VAMP-8 A/G) is merged with other SNPs, for example rs16988, rs1058601, rs3199256, rs17617682, rs56477175, and rs59206761. Therefore, this raises an assumption that rs1010 (VAMP-8 A/G), located at the 3 untranslated region, may have a pivotal role for VAMP-8 formation and may correlate with VAMP-8 levels in the circulation. Some studies had reported the association between VAMP-8 A/G gene polymorphism and the risk of CAD in some countries15-23. However, in our country, the study in this context has never been conducted.
Our present study aimed to investigate the correlation between VAMP-8 A/G gene polymorphism and the risk of acute myocardial infarction in our Hospital (Saiful Anwar General Hospital). This is the first report concerning the association between VAMP-8 A/G gene polymorphism and the risk of acute myocardial infarction in Indonesia and Southeast Asian countries.
Method
Study designs and patients
To assess the association between VAMP-8 A/G gene polymorphism and the risk of acute myocardial infarction, we performed a cross-sectional study at Saiful Anwar General Hospital, Malang, Indonesia from June 2013 to May 2014. The target population was all men subjects with acute myocardial infarction treated in our Hospital during the period. Men subjects aged ≥ 30 years with acute myocardial infarction were included in the study. Several parameters including clinical conditions, electrocardiography, myocardial enzyme, and angiography were used to confirm acute myocardial infarction diagnosis. While; patients with diabetes mellitus (DM), infection, impaired renal function, neoplasm, and subjects who disagree to give blood for the study were excluded from the study. Controls were healthy age-and-sex-matched subjects in our population (population-based). The blood samples from the peripheral vein were collected (10 ml), then were put in EDTA-coated tubes and kept cold at -140C. All patients had signed the informed consent before participating in the study. This study was approved by Ethical Committee of Universitas Brawijaya, Malang, Indonesia and carried out in accordance with Declaration of Helsinki for humans experiments.
VAMP-8 G/A genotype determination
Briefly, the genotype frequency was determined for all the cases and controls. The protocols were adapted from previous studies18-24 with some modifications. A set of primers (sense: 5´- GGG GGC TCC AAC TTT CTT CTC C and antisense 5´- CTT TGC CAC TGG TGC CTT CTC TTA) was designed to identify VAMP-8 A/G gene polymorphism. We amplified the DNA (Perkin Elmer 2400, Boston, USA) for 35 cycles and each cycle consisted of pre-denaturation at 95 °C for five minutes, denaturation at 980C for 20 seconds, annealing at 61 °C for 15 seconds, elongation at 72 °C for 45 seconds, and post- elongation at 72 °C for five minutes.
To determine VAMP-8 A/G gene polymorphism, Mae II enzyme with Restriction Fragment Length Polymorphism (RLFP) method was used. A 10 μL of total reaction volume contained 5.65 μl ddH2O, 1 μl Buffer Y, 0.35 μl Mae II enzyme, and 3 μl DNA PCR product. The mixture was then incubated at 65 °C for three hours. For G variant, the products of 328 bp and 166 bp were digested. While, for the A allele, the final product of 494 bp remained undigested. RFLP products were electrophoresed using a 2% agarose gel (Hoeffer, Holliston, USA), stained with ethidium bromide, and analyzed digitally using Gel Doc EZ System (Gel Doc, California, USA).
Statistical analysis
The association between VAMP-8 A/G gene polymorphisms and the risk of acute myocardial infarction was analyzed using multiple logistic regression. Statistically significant was considered if the P-value was less than 0.05. The Statistical Package of Social Sciences 17.0 software (SPSS Inc., Chicago, IL) was used to analyze the data.
Results
During the period, a total of 144 patients with acute myocardial infarction was treated in our Hospital. Of those, two patients were excluded because of aged under 30 years; 18 patients were excluded because of DM; five patients were excluded because of having infection; 11 patients were excluded because they had renal disease; three patients were excluded because of neoplasm, and we also excluded eight patients because they disagreed for the study. Finally, a total of 97 patients and 35 controls were included in the study. The exclusion pathway is described in Figure 1. The average age of acute myocardial infarction and control group was 57.5 and 57.6 years, respectively. Other parameters including smoking, hypertension, and lipid profile are presented in Table 1.
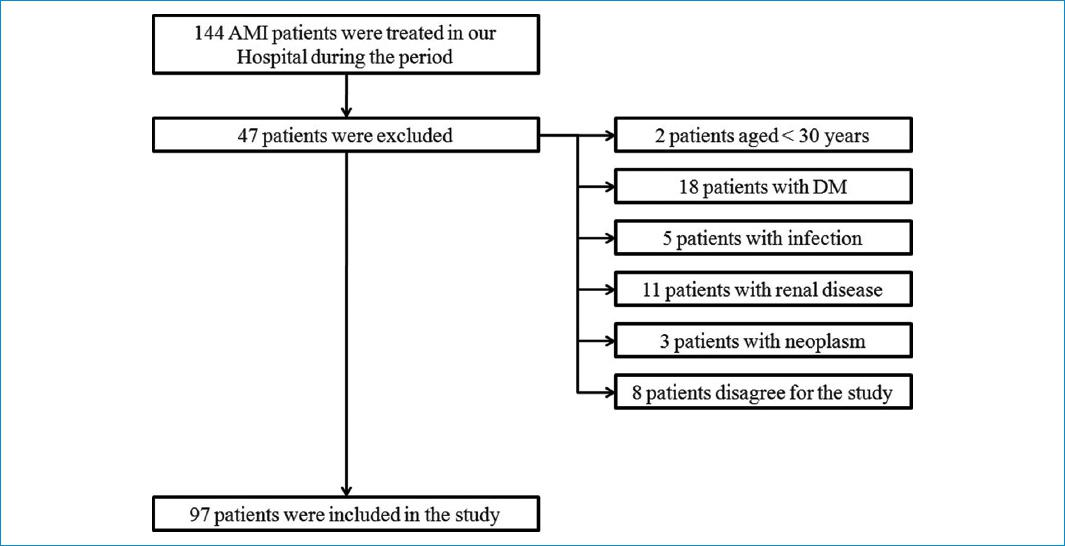
Figure 1 Inclusion and exclusion pathway in the study. AMI: acute myocardial infarction; DM: diabetes mellitus.
Table 1 Baseline characteristics and laboratory findings in the study
| Characteristics | Case (n = 97) | Control (n = 35) | OR | 95%CI | p |
|---|---|---|---|---|---|
| Age (years; mean ± SD) | 57.5 ± 11.1 | 57.6 ± 5.2 | 1.01 | 0.95 – 1.08 | 0.6820 |
| Smoker (n[%[) | 79 (81.4) | 16 (45.7) | 11.36 | 3.56 – 36.19 | <0.0001 |
| Hypertension (n[%[) | 43 (44.3) | 15 (42.9) | 1.50 | 0.53 – 4.22 | 0.4450 |
| Total cholesterol (mg/dl; mean ± SD) | 182 ± 45 | 196 ± 41 | 0.94 | 0.91 – 0.98 | 0.1380 |
| HDL (mg/dl; mean ± SD) | 66 ± 44 | 43 ± 10 | 1.08 | 1.03 – 1.12 | 0.1780 |
| LDL (mg/dl; mean ± SD) | 118 ± 41 | 119 ± 24 | 1.05 | 1.01 – 1.10 | 0.7640 |
| Triglyceride (mg/dl; mean ± SD) | 95 ± 73 | 94 ± 42 | 1.02 | 1.01 – 1.03 | 0.2400 |
Values are presented in mean ± SD or frequency (percentage); OR, odds ratio; CI, confidence interval; SD, standard deviation; HDL, high-density lipoprotein;
LDL, Low-density lipoprotein.
The frequencies of VAMP-8 A/G gene polymorphism in acute myocardial infarction and control groups are described in Table 2. RLFP for VAMP-8 A/G gene polymorphism is described in Figure 2. For acute myocardial infarction group, the frequency of GG, GA, and AA genotypes were 23, 44, and 30; respectively. While, for control group, the VAMP-8 A/G genotypes frequency were 5, 15, and 15 for GG, GA, and AA; respectively. Our genotype frequencies conformed with Hardy-Weinberg equilibrium both in case (X2 for HWE = 0.75) and control (X2 for HWE = 0.16). Our results showed that VAMP-8 A/G gene polymorphism was not associated with the risk of acute myocardial infarction.
Table 2 VAMP-8 A/G gene polymorphism between case and control
| VAMP-8 G/A genotypes | Case (n = 97) | Control (n = 35) | OR | 95%CI | p |
|---|---|---|---|---|---|
| GG (n[%]) | 23 (24) | 5 (14.3) | 1.87 | 0.65 – 5.36 | 0.247 |
| GA (n[%]) | 44 (45) | 15 (42.9) | 1.11 | 0.51 – 2.41 | 0.798 |
| AA (n[%]) | 30 (31) | 15 (42.9) | 0.60 | 0.27 – 1.32 | 0.204 |
Values are presented in frequency (percentage); OR, odds ratio; CI, confidence interval; SD, standard deviation; VAMP-8, Vesicle-associated membrane protein 8.
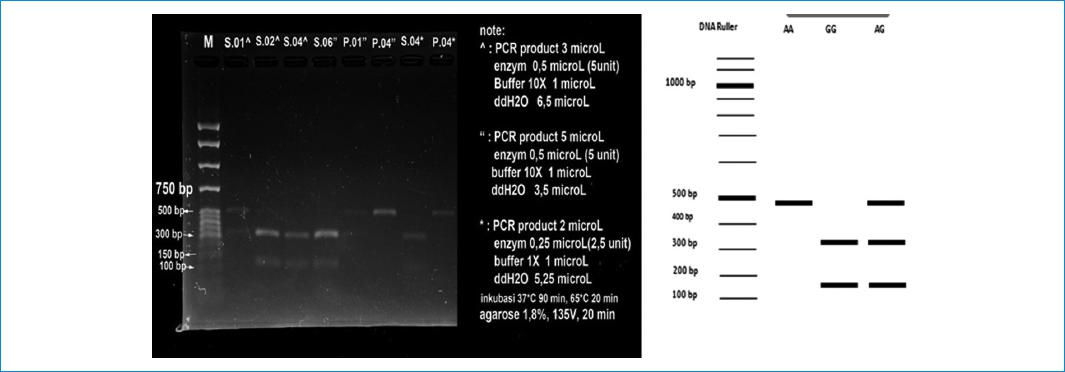
Figure 2 Restriction fragment length polymorphism of VAMP-8. This figure shows three genetic variant of VAMP-8 A/G (AA, GG, AG).
Furthermore, for subgroup analysis, we also analyzed several factors including smoking, hypertension, and early acute myocardial infarction in our study. The frequency of GG, GA, and AA was 22, 33, and 24; respectively for smoking group, and 1, 11, and 6; respectively for non-smoking group (Table 3). For acute myocardial infarction patients with hypertension (Table 4), the frequency of VAMP-8 A/G gene polymorphism was 10, 22, and 11 for GG, GA, and AA; respectively. While, for acute myocardial infarction patients without hypertension, the frequency was 13, 22, and 19 for GG, GA, and AA; respectively. For acute myocardial infarction patients aged under 55 years, the frequency of GG, GA, and AA was 13, 15, and 10; respectively. While, for acute myocardial infarction patients aged more than or equal to 55 years, the frequency was 10, 29, 20; respectively (Table 5). Our analysis found that no correlation was observed between VAMP-8 A/G gene polymorphism and those several factors.
Table 3 VAMP-8 A/G gene polymorphism in acute myocardial infarction patients between smoker and non-smoker
| VAMP-8 G/A genotypes | Smoker (n = 79) | Non-smoker (n = 18) | OR | 95%CI | p |
|---|---|---|---|---|---|
| GG (n[%]) | 22 (28) | 1 (6) | 6.56 | 0.82 – 52.31 | 0.076 |
| GA (n[%]) | 33 (42) | 11 (61) | 0.46 | 0.16 – 1.30 | 0.142 |
| AA (n[%]) | 24 (30) | 6 (33) | 0.87 | 0.40 – 1.67 | 0.807 |
Values are presented in frequency (percentage); OR, odds ratio; CI, confidence interval; SD, standard deviation; VAMP-8, Vesicle-associated membrane protein 8; acute myocardial infarction, acute myocardial infarction.
Table 4 The comparison of VAMP-8 A/G gene polymorphism in acute myocardial infarction between hypertensive and non-hypertensive patients
| VAMP-8 G/A genotypes | HT (n = 43) | Non-HT (n = 54) | OR | 95%CI | p |
|---|---|---|---|---|---|
| GG (n[%]) | 10 (23) | 13 (24) | 0.96 | 0.37 – 2.46 | 0.925 |
| GA (n[%]) | 22 (51) | 22 (41) | 1.52 | 0.68 – 3.42 | 0.307 |
| AA (n[%]) | 11 (26) | 19 (35) | 0.63 | 0.26 – 1.53 | 0.311 |
Values are presented in frequency (percentage); OR, odds ratio; CI, confidence interval; SD, standard deviation; VAMP-8, Vesicle-associated membrane protein 8; acute myocardial infarction, acute myocardial infarction.
Table 5 The association between VAMP-8 A/G gene polymorphism and early acute myocardial infarction
| VAMP-8 G/A genotypes | < 55 years (n = 38) | ≥ 55 years (n = 59) | OR | 95%CI | p |
|---|---|---|---|---|---|
| GG (n[%]) | 13 (34) | 10 (17) | 2.55 | 0.98 – 6.62 | 0.055 |
| GA (n[%]) | 15 (39) | 29 (49) | 0.68 | 0.30 – 1.54 | 0.351 |
| AA (n[%]) | 10 (26) | 20 (34) | 0.70 | 0.28 – 1.72 | 0.431 |
Values are presented in frequency (percentage); OR, odd ratio; CI, confidence interval; SD, standard deviation; VAMP-8, Vesicle-associated membrane protein 8; acute myocardial infarction, acute myocardial infarction.
Discussion
To date, genetic studies in the context of acute myocardial infarction have been widely reported. Of these, studies concerning VAMP-8 A/G gene polymorphism have a limited number. Nevertheles, theorethically, VAMP-8 is highly associated to acute myocardial infarction pathogenesis through platelet activation, secretion, and aggregation25. In our country, until now, no study reported VAMP-8 A/G gene polymorphism. Our present study reported VAMP-8 A/G gene polymorphism between acute myocardial infarction and control groups.
Our results found that VAMP-8 A/G gene polymorphism was not associated with the risk of acute myocardial infarction. Theoretically, VAMP-8 has a crucial role in platelet activation through stimulating platelet exocytosis and platelet-dense granule secretion10. This may lead to the fusion between granule membrane and platelet plasma membrane, and also fusion among granules26. This fusion is facilitated by membrane protein, called v-soluble N-ethyl maleimide sensitive factor attachment protein receptor (v-SNARE) (SNARE in granule) and t-SNARE (SNARE in targeted membrane). v-SNARE and t-SNARE may form two layers of heteromeric complex leading to membrane fusion and release granule content. As the results, platelet may be activated27. It has been widely known that platelet activation plays a pivotal role in the develpoment of acute myocardial infarction25. Therefore, theoretically, VAMP-8 A/G polymorphism may have the association with the risk of acute myocardial infarction. However, our study failed to support this theory. Further investigations with comprehensive methods are required to elucidate this correlation. Totally, based on searching in Pubmed and EMBASE, there were nine studies evaluating the correlation between VAMP-8 gene polymorphism and the risk of CAD. Our results were consistent with Luke et al.15, van der Net et al.16 and Akao et al.17 but contrast with Liu et al.18, Bare et al.19, Shiffman et al.20, Shiffman et al.21, Duan et al.22, and Ke-jun et al.23. Although some studies had proven the correlation between VAMP-8 gene polymorphism and the risk of CAD. However, it was not clear whether A or G allele had the impact on increasing the risk of CAD. Two studies had been conducted in the Chinese Han population, but showed different results. A study22 showed that A allele was correlated with an increased risk of CAD. While, another study18, although used inhomogeneous sample, found G allele. Moreover, although the association between VAMP-8 gene polymorphism and the risk of CAD was also reported by Bare et al.19, however, they had no control. They compared their results with control of other studies. Therefore, some factors including different population and region may affect the correlation, and study bias remained to be considered. Furthermore, some studies16,23 included female with or without post-menopause subjects. As well known that menopausal factors have proven to contribute in increasing the risk of CAD28, and therefore this factor may cause study bias. In addition, some studies data were not presented in hardy-Weinberg equilibrium and fulltexts were not available20,23. Therefore, the data could not be analyzed further. In our present study, we designed our study with eliminating these limitations factors. Therefore, we expected that our results might provide the better outcome. Moreover, due to these reports remain conflicting, we calculated odds ratio and 95 confidence interval (OR 95%CI) of five studies, including our results, to conclude the association. The pooled calculation found that no correlation was observed between VAMP-8 gene polymorphism and the risk of CAD (Fig. 3A). However, our calculation is not the final. In the near future, we expect that there will be the studies evaluating this topic with specific design especially meta-analysis.
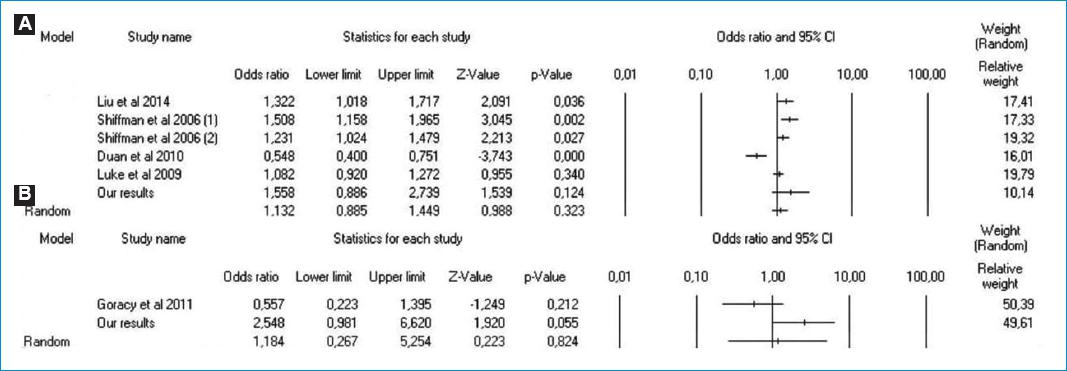
Figure 3 A: Forest plot concerning the correlation between VAMP-8 G/A gene polymorphism and the risk of CHD (G vs. A). B: Forest plot the comparison of patients with acute myocardial infarction between subjects < 55 years and ≥ 55 years (GG vs. GA + AA).
For sub-group analysis, we also evaluated the correlation between VAMP-8 gene polymorphisn and several factors including smoking, age, and hypertension among acute myocardial infarction patients. Theoretically, these factors have pivotal role in VAMP-8-related to acute myocardial infarction. During this time, no study reports the direct correlation between VAMP-8 and smoking. However, the possible mechanism has been proposed. In smoker patients, FXIII is up-regulated. The adhesion of activated platelet to FXIII, mediated by GpIIbIIIa receptors, is one of platelet aggregation pathways. While, platelet secretion and activation are regulated by VAMP-8. This process is the begining of thrombus formation in CAD patients29. However, our results showed otherwise, no correlation was observed between VAMP-8 A/G gene polymorphism and smoking among acute myocardial infarction patients. Moreover, for the correlation between VAMP-8 and hypertension, it has been reported that, by mediating normal granule maturation, VAMP-8 inhibits renin release. Renin-angiotensin-aldosteron-system is the pathway responsible for hypertension30,31. This means that elevated level of VAMP-8 has protective role against hypertension. In this context, we also failed to show the correlation. In the previous studies15-23, these factors were not involved in the analysis. Therefore, the correlation between VAMP-8 gene polymorphism and both smoking and hypertension among acute myocardial infarction patients was unknown. For this reason, we could not compare our outcome to the previous studies, either systematically or naratively. Moreover, data in the literature was not enough to elaborate the possible reason.
Furthermore, for the association between age and VAMP-8 A/G gene polymorphism, several studies21,24 had reported the correlation between early acute myocardial infarction and VAMP-8 A/G gene polymorphism. This correlation is assumed that VAMP-8 may cause the reduction of stem-loop structure stability. As the results, this leads to plaque destabilization which triggers to early acute myocardial infarction21. Although they showed that VAMP-8 A/G polymorphism was associated with early acute myocardial infarction, however, whether the A or G allele correlating to the risk of early acute myocardial infarction is still inconclusive. Shiffman et al.21 showed that G allele was found to be correlated with the risk of early acute myocardial infarction, while Goracy et al.24 found A allele. Moreover, the definition of early acute myocardial infarction in the previous studies was unclear, ranging from less than 45 to 60 years old21,24,32,33. For this reason, we defined early acute myocardial infarction as subjects with age less than 55 years old, and our results found that no association between early acute myocardial infarction and VAMP-8 A/G polymorphism. Our results were contrast with those previous studies21,24. Due to this difference, we combined our data with Goracy et al.24, and we also found that no correlation was observed between early acute myocardial infarction and VAMP-8 A/G polymorphism (Fig. 3B). However, early acute myocardial infarction is complex involving several factors including smoking, systolic hypertension, dyslipidemia, history of diabetes, and psychosocial factors32,34. Therefore, for the future studies, we suggested that these factors are controlled to determine the better outcome.
Our study had several limitations. First, some pivotal factors in thrombosis pathway including platelet function and the function of VAMP-8 expression were not measured. Second, study bias due to small sample size could drive to false negative findings. Third, samples in our study were only recruited from Saiful Anwar General Hospital. Fourth, several factors that might govern the gene polymorphism were not assessed, including environment, race, and lifestyle. Fifth, until now, the precise SNP affecting the level of VAMP 8 in the circulation is not well understood. Therefore, it might be difficult to assess the role of VAMP 8 gene polymorphism on the risk of acute myocardial infarction. Further studies with larger and well-characterized population are required to determine real effect of VAMP 8 gene polymorphism on the risk of acute myocardial infarction.
Conclusions
Our data suggest that, in our population, VAMP-8 A/G gene polymorphism is not associated with the risk of acute myocardial infarction. Moreover, VAMP-8 A/G gene polymorphism also has no correlation with acute myocardial infarction patients with smoking habits, hypertensive subjects, and premature acute myocardial infarction. Our results may contribute the better understanding concerning the VAMP-8 A/G gene polymorphism in acute myocardial infarction. However, further studies are required to determine the better outcome with eliminating the limitation factors.














