Introduction
Takotsubo syndrome is a potentially reversible acquired cardiomyopathy secondary to emotional or physical stress, which typically presents as an acute affectation of the left ventricular myocardial contractility. However, the affectation can be right-sided or even biventricular1. Takotsubo has a dramatic clinical presentation with a sudden offset of chest pain, breathlessness, or collapse, leading the patient to believe that she is suffering a myocardial infarction. Like acute coronary syndrome patients, takotsubo patients are at higher risk of all-cause and cardiac-specific death and severe consequences as cardiogenic shock or ventricular rupture2,3.
The similarity between takotsubo and acute coronary syndrome goes beyond the clinical presentation, comorbidity is not rare4, and they share common findings in ECG and biomarkers that difficult their differentiation even to paramedics and emergency service physicians5. Differential diagnosis often requires invasive angiography to identify coronary artery patency and myocardial ballooning, its most characteristic objective findings6. Despite these similarities, takotsubo has different pathophysiology, which is not entirely understood as related literature is scarce and mainly based on animal models.
The human pathophysiology of the takotsubo cardiomyopathy is still a topic of debate. The most accepted hypothesis states that an exaggerated sympathetic stimulation leads to a cardiotoxic discharge of circulating catecholamine7. However, it is not clear if this is causative, consequential, or an epiphenomenon of the takotsubo physiology8. In the line of the catecholamine hypothesis, recent neuroimaging studies have reported alterations in brain structures and networks involved in emotional control and autonomic regulation that may compromise the sympathetic adrenomedullary and the hypothalamic-pituitary-adrenal axes, which regulate the liberation of catecholamines9,10.
Neuroimaging findings may be relevant to the clarification of our understanding of the pathophysiology of the takotsubo cardiomyopathy and the neurocardiology of stress-related cardiomyopathies in general. However, to the date, there is no systematic review that synthesizes functional neuroimaging results in patients with takotsubo. Therefore, the purpose of this study is to review the available evidence of brain functional connectivity in takotsubo cardiomyopathy patients.
Methods
Protocol and registry
This was a systematic review of observational studies. The review protocol was developed considering guidance from the Cochrane handbook for systematic reviews of interventions11 and was registered in PROSPERO repository (CRD42020149634). This article structure follows the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta Analyses)12. This study was approved by the CES University Ethical Review Board.
Eligibility criteria and sources of information
Studies were considered eligible if conducted in: 1) (Population) Adults (over 18 years) with takotsubo syndrome diagnosed by a cardiologist or by a psychiatrist, in whom the results of 2) (Intervention) resting state or functional connectivity secondary to paradigm functional Magnetic Resonance Imaging (fMRI) were compared to 3) (Comparator) healthy controls or patients with other cardiomyopathies (diagnosed by a cardiologist), in terms of 4) (Outcome) connectivity changes in specific regions or networks of the brain 5) (Time) evaluated up to 36 months after the event. 6) (Study) Observational studies and case (series) reports published in English, Spanish, Italian, and French until September 9, 2019, in MEDLINE, LILACS, Ovid (Cochrane), Scopus, ScienceDirect, Clinical Trials, and PROSPERO were considered eligible. A manual reference search among the included studies was conducted.
Search strategy and study selection
Search strategy performed in MEDLINE was (fMRI OR "magnetic resonance" OR connectomics OR "default mode network" OR DMN OR "resting state" OR "task activation network" OR "Independent component analysis" OR ICA OR "resting state network" OR voxel OR voxels OR "functional connectivity") AND (takotsubo[Title/Abstract] OR "apical ballooning"[Title/Abstract] OR "stress-induced cardiomyopathy"[Title/Abstract] OR "Gebrochenes-Herz"[Title/Abstract] OR "stress cardiomyopathy"[Title/Abstract] OR "broken heart syndrome"[Title/Abstract]). The same strategy was used in other sources, adjusting for syntaxis difference.
All study registries identified were recorded in a Microsoft Excel spreadsheet, duplicate publications were eliminated. LO, AM, and JV screened titles and abstracts, excluding those not pertinent to the PICO question. DR and CR reviewed the complete text of eligible articles, excluding those not fulfilling eligibility criteria. The inclusion of studies in the narrative synthesis was defined by consensus among all authors.
PICOS variables and data collection process
All authors performed a full-text review. Information was extracted and registered in an Excel spreadsheet by LO, AM, and JV, discrepancies were solved by full-group review of the document. Extracted characteristics were: population: sample size, age, and sex distribution, clinical diagnosis (emotional or physical trigger and comorbidities), geographical or institutional settings. Intervention: default mode network, resting-state, or functional connectivity secondary to paradigm (memory, pain, language, motor, etc.). Comparator: sample size, age and sex distribution, clinical diagnosis (other myocardiopathy or healthy control), and geographical or institutional settings. Outcome: Specific connectivity or activity measure reported. Study: first author surname, year of publication, and study design.
Risk of bias assessment
For each included study, DR and CR evaluated the study-level risk of bias; differences were solved by consensus between both evaluators. Evaluation of observational studies was carried out with the Newcastle-Ottawa Scale (NOS)13. We present the study-level evaluation of each domain for individual studies and summarize the risk of bias across studies.
Summary measures and synthesis of results
The study-level results are presented in a summary table. We highlight the specific regions of the brain with statistically significant (p < 0.05) evidence of altered connectivity. A narrative synthesis was performed integrating study findings according to increased or decreased connectivity of specific subnetworks and their role in autonomic regulation. This narrative is complemented with a graphical representation of the most relevant findings in resting-state fMRI. No additional analyses were performed.
Results
Study selection
The database search retrieved 725 registries. After eliminating duplicates, 600 titles and abstracts were screened and 592 were excluded for not being relevant to the review question. The remaining eight full texts were reviewed; three were excluded from the study, two for relevance14,15, and one that used SPECT16; five studies where included in the study (Fig. 1).
Risk of bias of included studies
In general, the studies presented a low risk of bias, and the five studies were considered for qualitative synthesis (Table 1). We could not determine the representativeness of the cases in the studies by Pereira17 and Sabisz18. For the latter study, we either could not determine the source of the controls. Concerning comparability, all studies except the one by Pereira17 matched controls to cases by age; the five studies included female cases only. Despite presenting statistical analyses of differences between cases and controls in potential confounders, no study adjusted for them in the statistical analyses. Klein and Pereira had missing data for cases, mainly for reasons related to contraindication or technical issues.
Table 1 Risk of bias of included case-control studies (Newcastle-Ottawa Scale)
| Study | Selection | Comparabilitya | Exposure | Total score |
|---|---|---|---|---|
| Silva 2019 | **** | * | *** | 8/9 |
| Templin 2019 | **** | * | *** | 8/9 |
| Klein 2017 | **** | * | ** | 7/9 |
| Sabisz 2016 | ** | * | *** | 6/9 |
| Pereira 2016 | *** | ** | 5/9 |
aMost important factors: age and sex. Additional factors: cognitive function, depression and anxiety.
Study characteristics and main findings
Table 2 presents a summary of the specific characteristics and main findings for each study. Figures 2 and 3 present a graphical representation for resting-state and autonomic challenge, respectively.
Table 2 Main characteristics and findings of included case-control studies
| Author, Year | Age | Time since the event (months) | fMRI Methods | Paradigm | Main findings |
|---|---|---|---|---|---|
| Silva, 2019 | 58.6 ± 7.4 | Mean: 36.0 | GTA NBS |
Resting state | ↑ Bilateral hippocampus |
| Topical cold stimulation | ↑ Right insula; right medial temporal gyrus; right superior occipital gyrus; left amygdala; bilateral cerebellum; left putamen; right inferior temporal gyrus; right parietooccipital junction | ||||
| Templin, 2019 | 64.4 ± 15.0 | Median: 12.6 | NBS ROI |
Resting-state | ↓ Parasympathetic network: right amygdala; bilateral hippocampus; bilateral superior and medial temporal gyrus; left motor cortex; left supra-marginal gyrus; left cerebellum ↓ Sympathetic network: bilateral middle cingulum; left prefrontal cortex; bilateral cerebellum; bilateral supra-marginal gyrus ↓ Default mode network: bilateral hippocampus; left para-hippocampal gyrus; bilateral ventral and dorsal prefrontal cortex; bilateral posterior cingulum; left temporal pole; bilateral inferior parietal lobule; bilateral temporoparietal junction ↓ Whole-brain: left anterior insula; left posterior cingulum; bilateral medial frontal-orbital cortex; left medial temporal gyrus; right globus pallidus; bilateral cerebellum |
| Klein, 2017 | 65.3 ± 14.3 | Median: 5.6 | ReHo | Resting-state | ↓ Bilateral para-hippocampal gyrus*; left amygdala; left paracentral lobe; bilateral supplementary motor cortex; left superior parietal lobe*; left insula* ↑ Right paracentral lobe*; Left hippocampus; right precentral gyrus; left fusiform gyrus |
| Sabiz, 2016 | 62.0 ± 7.0 | Min: 12 Max: 18 |
ICA ALFF |
Resting-state | ↑ Bilateral precuneus↓Bilateral ventromedial prefrontal cortex |
| Pereira, 2016 | 67.0 ± 12.0 | Not reported | ROI ALFF |
Valsalva Maneuver | ↓ Left Insula (basal); Bilateral amygdala↑Right Insula (basal); Left Insula; Right Insula; Right hippocampus |
| Cold exposure | No significant variations |
GTA: Graph theory analysis; NBS: Network-based statistics; ROI: Regions of interest; ICA: Independent component analysis; ReHo: Regional homogeneity; ALFF: Amplitude of low-frequency fluctuation. *Regions with higher positive predictive value for takotsubo patients. ↓: Decreased connectivity, ↑: Increased connectivity.
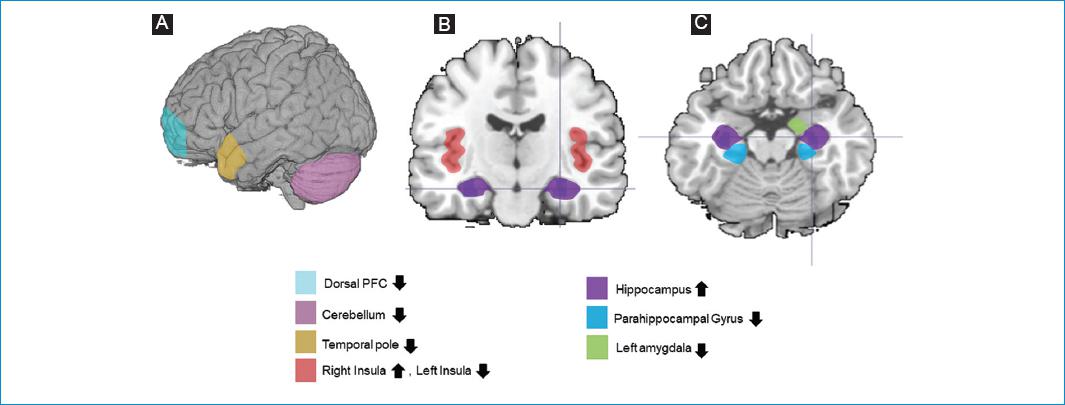
Figure 2 Brain alterations described in resting-state fMRI analyses (selection of main findings). A: external surface; B: coronal plane; C: transverse plane. ↓ Decreased connectivity, ↑ Increased connectivity.
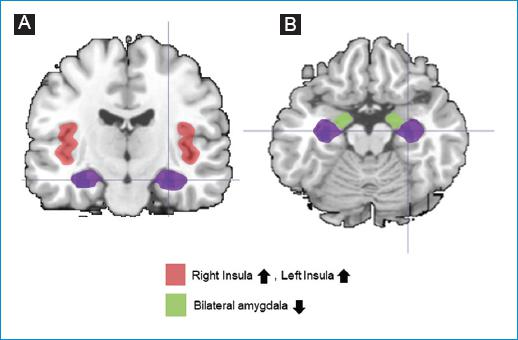
Figure 3 Brain alterations described in autonomic challenge (Valsalva) fMRI analyses (selection of main findings). A: coronal plane; B: transverse plane. ↓ Decreased connectivity, ↑ Increased connectivity.
Silva et al.19 performed resting state and under stressful (topical cold) stimulation fMRI in eight prevalent female cases (mean age 58.6 years, SD: 7.4) from the Hospital of Braga, and in eight sex and age-matched controls (no specification about their source). The mean time between the event and the fMRI was 36.0 months. Cases and controls showed no significant differences in years of education, anxiety (HADS) or perceived stress (PPS-10). However, cases showed a significantly higher score of depression (HADS). The authors used network-based statistics and graph theory analysis to analyze both resting state and topical cold stimulation fMRI. For the latter, connectivity networks were gathered 120 seconds pre and 60 seconds post-stimulation.
The authors found increased connectivity (cases vs. controls) in a network that included the left amygdala and the right Insula. Under resting-state fMRI, the authors identified a network composed by the left anterior Insula, left anterior cingulate cortex, superior temporal cortices, left inferior frontal cortex, left hippocampus, and left Parahippocampal cortex. In this network, the higher number of enhanced connections was observed in the right superior temporal cortex; there was no evidence of decreased connectivity in any network. Under the autonomic (cold stressor) challenge, the highest number of connections was observed in the left amygdala.
Templin et al.9 performed resting-state fMRI in 15 prevalent female cases (mean age 64.4 years, SD: 15.0) from the InterTAK registry (University Hosptial Zurich), and in 39 sex and age-matched healthy controls from the IHAB healthy aging study database, between 2013 and 2014. The median time between the event and the fMRI was 12.6 months. Cases and controls showed no significant differences in handedness, cognitive function (MMSE), anxiety, or depression (HADS). Altered functionality was analyzed with network-based statistics of four networks: 1) Sympathetic network, 2) parasympathetic network, 3) default mode network, and 4) whole-brain analysis.
The authors found reduced functional connectivity (cases vs. controls) in subnetworks associated to both parasympathetic (right amygdala, left and right hippocampus, left and right middle and superior temporal gyrus, left primary motor cortex, left supramarginal/angular gyrus, and the left cerebellum) and sympathetic networks (left and right amygdala, left and right middle cingulate gyrus, left dorsolateral prefrontal cortex, left and right cerebellum, and the left superior parietal lobule/supramarginal gyrus). This reduced functional connectivity was also observed within the default mode network (left and right hippocampus, left parahippocampal gyrus, bilateral dorsal and ventral medial prefrontal cortex, left and right posterior cingulate cortex, left temporal pole, bilateral posterior inferior parietal lobule, and the left and right temporoparietal junction). The whole-brain analysis confirmed the specificity of limbic regions.
Klein et al.20 performed resting-state fMRI in 20 prevalent female cases (mean age 65.3 years, SD: 14.3) from the International takotsubo Registry, and in 19 sex and age-matched healthy controls from the International Normal Aging and Plasticity Imaging Center database. One case was excluded because of incidental brain anomalies and three cases for image-related methodological reasons. The median time between the event and the fMRI was 5.6 months. Cases and controls showed no significant differences in handedness, cognitive function (MMSE), anxiety, or depression (HADS). Connectivity differences were analyzed with multivariate machine learning algorithms (the Pattern Recognition for Neuroimaging data Toolbox -ProNTo).
The authors found anatomical and neurophysiological alterations in brain regions involved in the systems of autonomic control and emotional processing. Under resting-state fMRI, the bilateral parahippocampal gyri, paracentral lobule, superior parietal lobule, and left Insula showed the highest predictive values for classification of cases and controls. For voxel-based morphometry analyses, the left precentral gyrus, both paracentral lobule, bilateral supplementary motor areas, inferior temporal gyri, and superior parietal lobe showed the most relevant results. In a following research letter21 (not included as it is not an original article), the group reported anatomical differences in elements of the limbic network comprising the Insula, amygdala, cingulate cortex, and hippocampus.
Sabisz et al.18 performed resting-state fMRI in 13 prevalent female cases (mean age 62.0 years, SD: 7.0) and in 13 age and sex-matched healthy controls. There is no explicit declaration of the source of participants, neither an explicit comparison of cases and control for potential confounders. Cases were taken to fMRI between 12 and 18 months after the event. Functional connectivity differences were analyzed on the default mode network. The authors found increased connectivity areas (cases vs. controls) in the precuneus and decreased connectivity areas in the ventromedial prefrontal cortex.
Pereira et al.17 performed fMRI in four prevalent female cases (mean age 67.0 years, SD: 12.0), and in eight healthy controls from the general population of the region of Braga (Portugal) (mean age 66.0 years, SD: 5.0). There is no explicit declaration of the source for cases, neither of the time between the event and examination. The original protocol considered 10 cases. According to authors, six were excluded because contraindications for fMRI3, one was lost during follow-up, and two that refused to participate. There is no explicit comparison of cases and controls for potential confounding variables. Functional connectivity was evaluated under two autonomic challenges: cold exposure and Valsalva maneuver. The authors found altered response of the insular cortex, the amygdala, and the right hippocampus to the autonomic challenge (the Valsalva maneuver). The authors found that patients had a statistically lower volume in the left amygdala than healthy controls. However, no significant volumetric changes were reported.
Synthesis of results
Despite significant differences in the characteristics of participants, small sample sizes, and risk of bias in comparability (potential confounding), the five studies point toward relatively similar significant findings and conclusions.
For Resting-state fMRI, at least two studies found significant differences for the hippocampus9,19,20, the Insula, the amygdala, and the parahippocampal gyrus9,20. Decreased connectivity was observed in all regions, except for the studies of Silva19 and Klein20, who found increased connectivity of the hippocampus. The Insula presented left-lateralized altered connectivity in both studies9,20. The laterality is not clear for the hippocampus, the amygdala, or the parahippocampal gyrus.
For Task fMRI, at least two studies found significant differences for the Insula, the superior occipital gyrus, and the amygdala17,19. Decreased connectivity was observed in all regions, except for the studies of Pereira17, who found decreased connectivity of the amygdala under the Valsalva maneuver. The superior occipital gyrus presented right-lateralized altered connectivity in both studies17,19. The laterality is not clear for the Insula and the amygdala.
The medial temporal gyrus and the cerebellum presented significant findings in one resting-state fMRI9 and one task-fMRI study19. The hippocampus (parasympathetic and default-mode), the cerebellum, and the supramarginal gyrus (parasympathetic and sympathetic) showed significant results in at least two of the networks analyzed by Templin9.
Discussion
This study aimed to critically review the evidence of brain functional connectivity in takotsubo cardiomyopathy patients, to contribute to a better understanding of the potential interplay between brain and heart in the pathophysiology of stress-related cardiomyopathies. Main findings from five included studies of overall good methodological quality highlight functional connectivity alterations of the Insula and the Amygdala in takotsubo patients, both in resting-state and task fMRI. However, the specific nature of those alterations (i.e., laterality and direction) is not clear. Consequently, those conclusions will require a prompt update as the number of available studies and their sample sizes is low, and they present relevant methodological differences in participants characteristics as the time between the event and the fMRI, event trigger, and age, and in fMRI protocols.
Due to its ability to modulate heart rate and conduction velocity, as well as cardiac contractility, the autonomic nervous system plays a central role in neurocardiac dysregulation22. After reviewing studies of autopsied hearts of healthy subjects, Pasupula et al.23 concluded what they called a "constellation of catecholamine surge with hypokinetic left ventricle apex and functional hyperkinetic left ventricle base." According to the authors, "poor cholinergic nerve distribution in the left ventricle apex as an associated factor augmenting microcirculatory dysfunction due to an unopposed Adrenergic Nerve activity from the catecholamine surge" may explain myocardial ballooning, the characteristic phenomenon of the takotsubo cardiomyopathy23.
Borodzicz et al.24 in their review of the role of the autonomic nervous system in takotsubo syndrome stated that this increase of catecholamine might be produced by two mechanisms of the central nervous system: the sympathoneural, causing a local myocardial discharge of norepinephrine, or the adrenomedullary hormonal, increasing blood level of catecholamine. The authors concluded that the available studies confirm the role of the autonomic nervous system, with a predominant report of activations of the sympathetic nervous system and fewer reports of alterations in the parasympathetic24. However, the specific autonomic nervous system alterations explaining changes in the modulation of catecholamine are not clear25-27.
As a prominent endpoint of the ascending afferent system and having significant efferent projections involved in descending autonomic cardiovascular modulation, the Insula has a special place in the role of the autonomic nervous system in cardiac dysregulation22. Classically described as a limbic integration cortex, recent studies suggest that the Insula is physiologically involved in multiple functions of human cognition and behavior28, and pathologically involved in multiple psychiatric and neurological conditions experiencing abnormal subjective feeling states, cognitive deficits, and motivational deficits29. Osteraas and Lee´s chapter on neurocardiology provide a review of proposed mechanisms by which the Insula may cause various pathologic neurocardiac findings22.
Evidence is scarce. However, the clinical implications of functional connectivity alteration in takotsubo patients are glimpsing on the horizon. For instance, Madias30, in a comment on a paper by Yu et al.31, highlighted the potential applicability of optogenetic neuromodulation for the monitoring and therapy of patients with ventricular arrhythmias, atrial fibrillation, angina, and takotsubo syndrome. Optogenetic is an approach with potential applications for cardiovascular research32.
These contemporary discussions highlight the relevance of brain functional connectivity studies in takotsubo patients, like those included in this systematic review. However, the study-level and review-level limitations restrict the conclusions from this review and could be considered in the design of the future fMRI studies in takotsubo patients.
The sample sizes were small and no study gave proper justification of how they arrived to it. This situation is common in neuroimaging studies. A systematic review of sample size calculations in fMRI studies identified that only 1% of a random sample of scientific articles reported sample size calculations33. The analysis of fMRI involves several variables and a need to correct for multiple comparisons and sample size around 20-30 subjects, like those of included studies, provide low statistical power, and consequently underrepresent the true effect sizes and are highly susceptible to variation in subsequent replications34. Thus, several regions of the brain may exhibit relevant functional alterations in takotsubo patients but cannot be statistically significant in small sample sizes. This is particularly relevant to clarify the directionality (increase or decrease) and laterality of alterations. Even when observed differences may be explained by between-study differences in the characteristics of included participants, instability of estimations due to small sample size cannot be discarded.
Inclusion criteria or subgroup analysis by triggers were not considered in several studies, and all cases were female. According to a recent systematic review of case reports, a physical trigger factor is more common than an emotional trigger factor in takotsubo syndrome, with differences by sex35. Prognostic outcomes like in-hospital mortality differ according to the trigger factor and sex36. In a similar fashion, alterations in brain connectivity may depend on the nature of the trigger an even on sex, but specific studies are required for clarification.
Conclusion
Brain connectivity alterations involving relevant elements of the autonomic nervous system like the insula and the amygdala provide evidence in favor of the role of functional networks in the neurocardiology of stress-related cardiomyopathies. However, it is not possible to determine if it plays a causal or a consequential role.