Introduction
Aortic valve repair has become an attractive alternative to aortic valve replacement in selected patients with aortic insufficiency (AI) during the last decades2. Surgical correction of AI by circumclusion and bicuspidization was already attempted before the advent of cardiopulmonary bypass3,4. In 1960 Starr and colleagues reported the first technique for aortic valve repair for aortic valve prolapse5. With the implementation of percutaneous balloon aortic valvuloplasty in the 1980s cardiac surgeons became more and more involved in aortic valve repair. Balloon- induced injuries in young patients required more often immediate repair. Therefore a broad variety of surgical techniques have been developed during the following decades with different short- and long- term outcomes6.
Although implementation of aortic valve repair into daily surgical practice has evolved, it is still reserved to high- volume and specialized centers. Reason for this may include the complexity of aortic valve repair surgery, the greater incidence of stenosis relative to regurgitation and the excellent long-term results with available aortic valve prosthesis. However, aortic valve replacement in the younger adults is still associated with morbidity, reduced quality of life and eventually reduced life-expectancy compared to the general matched population7. The use of mechanical valves exposes the patient to lifelong need for anticoagulation, risk of thromboembolic events and valve thrombosis. The risk for major bleeding is significant with approximately 1% per year8. Also, anticoagulation in females in child-bearing age has its own risks and is complex. The use of biological prostheses in younger age is associated with early degeneration and need for reintervention and may increase the risk for endocarditis9. Therefore, the aim of this review is to describe the important principles of aortic valve repair including anatomy, surgical techniques and outcomes. Second, we address the key steps in how to start your own aortic valve repair program.
Anatomy of the aortic valve and the aortic root
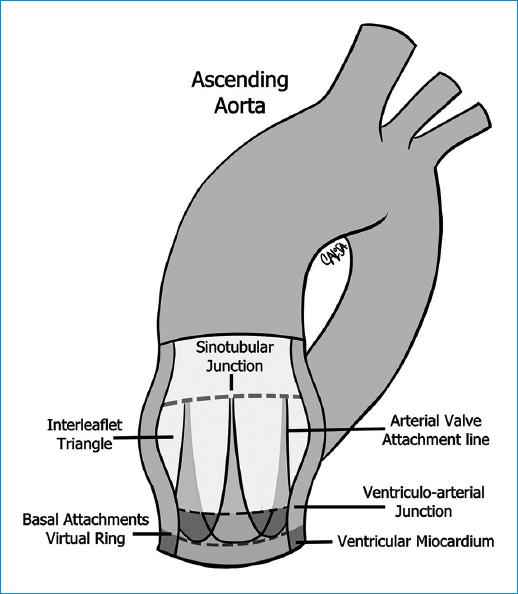
Knowing and understanding the anatomy of the aortic valve and root, and its complexity and interdependence is the key to a good repair. The aortic root can be defined in a simpler way as the union of the outflow tract of the left ventricle and the aorta, located between the sino-tubular junction (STJ) superiorly and the aorto-ventricular junction (AVJ) inferiorly. However, it has multiple components as the STJ, the sinuses of Valsalva, the leaflets, the commissures, the interleaflet or subcommissural triangles and the aortic anulus (AA), and functions. The aortic root supports the leaflets of the aortic valve, guides the unidirectional flow of large volumes of blood with minimal resistance while maintaining laminar flow, prevents the aortic valve leaflets from impacting the aortic wall, facilitates the closure of the aortic valve of and improves the coronary flow in diastole10 (Fig. 1).
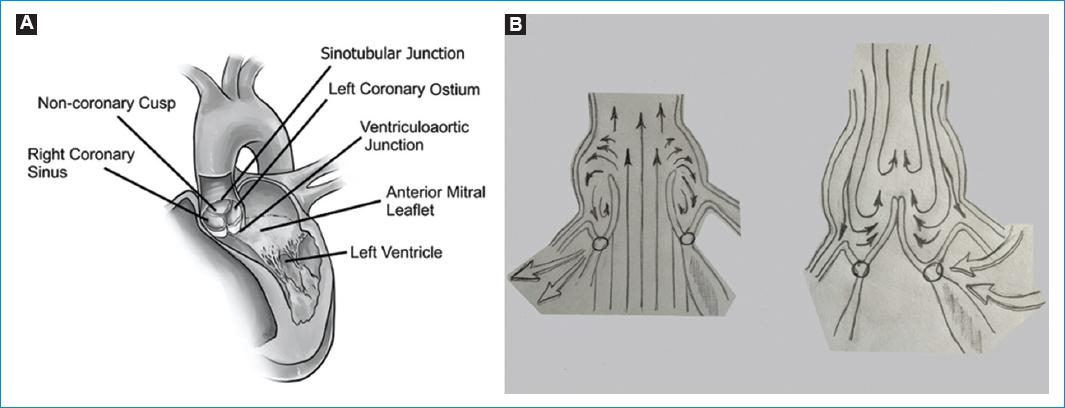
Figure 1 A: aortic root. B: schematic diagram of Da Vinci´s sinus currents and eddies in sistole and dyastole.
Aortic valve leaflets
The aortic valve is composed of three leaflets or cusps. Nevertheless, there are some variations which are important (Fig. 2). Each leaflet has a semilunar shape and is divided in three components: free margin, body and insertion or base11 (Fig. 3). The free margin is the coaptation surface divided in nodule of Arantius, central fibrous structure, and lunulae, lateral to it9. The leaflet insertion forms a crown shaped structure. The lowest point of this insertion is the nadir and the point at which the free margin of the leaflet joins its base is the commissure11.
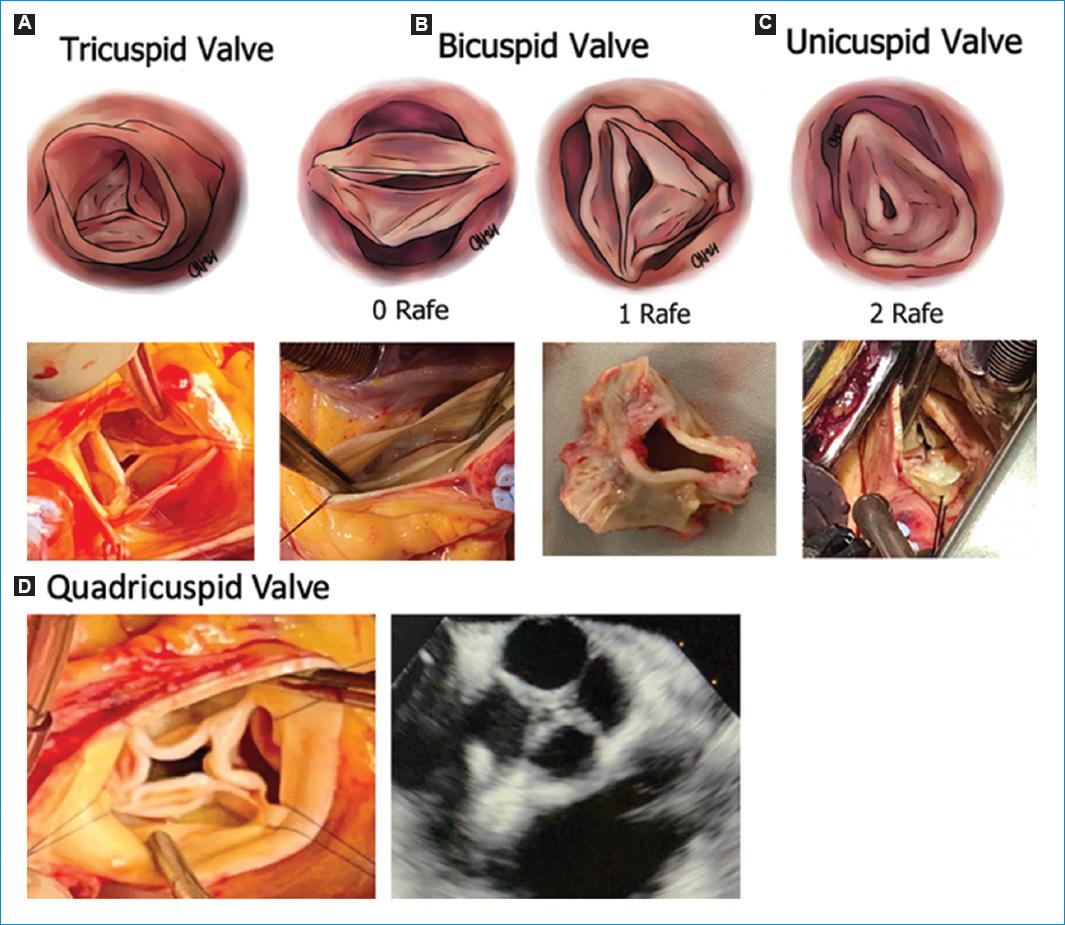
Figure 2 Valve type. A-C: define by the number of cusps: Tricuspid (3 completely developed commissures), Bicuspid (2 completely developed commissures and 0 (type 0) or 1 (type 1) raphe), unicuspid (1 completely developed commissure and 2 raphes). D: quadricuspid valve (Rare, 4 completely developed comissures).
The "Annulus Concept" and interleaflet triangles
The word annulus (AA) implies a circular structure but there is no histological or anatomical structure that fits this description in the aortic root. Given the dynamics of the aortic root, we have to consider two different concepts (Fig. 3)11:
- Virtual basal ring: The circumference defined by the nadirs of the crowned-shaped insertions, referred for repair purposes as aortic annulus.
- Ventriculo - arterial junction (VAJ): The junction between the left ventricular myocardium and the arterial structure of the aorta.
The interleaflet triangles are the triangles formed under each commissure. The triangle beneath the right-coronary and non-coronary sinuses is in direct continuity with the membranous septum and contains the His bundle. The injury of this area can lead to conduction abnormalities than can require a pacemaker implantation. Under the left and non-coronary triangle, the aorto-mitral curtain leads to the anterior mitral valve leaflet (Fig. 3)11.
Sino-tubular junction and sinuses of valsalva
The tubular structure immediately above the commissures and towards the ascending aorta is called STJ. The STJ separates the aortic root from the ascending aorta12. The three bulges, that are located between the insertion of the leaflets and the STJ are called sinuses of Valsalva11.
Geometric anatomy for valve repair
The aortic root has a geometry that makes its components interdependent. To make the aortic valve repair more reproducible and durable, geometric anatomy of the valve has been developed with mandatory measures that will guide the repair (Fig. 4):
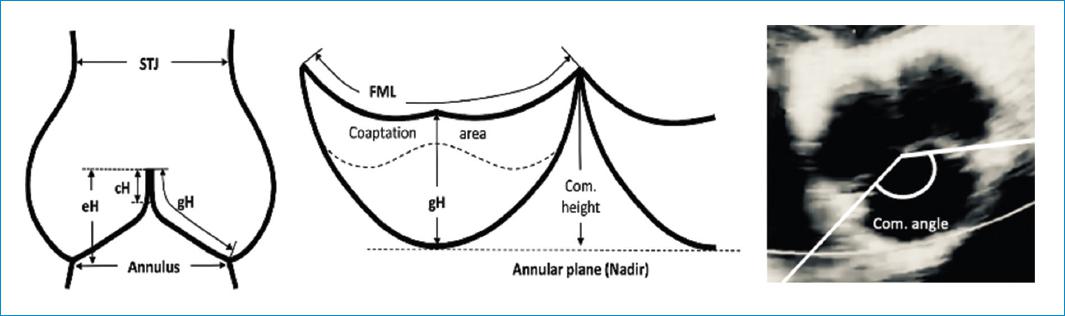
Figure 4 Diagram of aortic valve and root illustrating the different anatomical measurements used in aortic valve repair. cH: coaptation height, Com. angle: commissure angle, Com. height: commissure height, eH: effective height, FML: free margin length, gH: geometric height, STJ: sinotubular junction diameter.14
- Commissure orientation is defined as the angle formed by the lines joining the commissures to the central axis of the valve, in the non-fused side. This angle varies between 120o in tricuspid and very asymmetric bicuspid valves and 180o in symmetric bicuspid valves13.
- The AA will be defined in these cases as the virtual basal ring which is the circumference passing through the nadir of the aortic cups. This diameter is 10-20% larger than the STJ diameter in young patients. With age the elastic fibers in the arterial wall decreases and the STJ dilates and its diameter tends to equals the aortic annulus diameter.
- The effective height (eH) is the distance from the AA to the middle of the free margin of the cusp. The normal eH in adults is 9mm
- The geometric height (gH) is the distance from the nadir to the middle of the free margin. In adults, the cusp is considered retracted if the gH is ≤ 16mm in tricuspid valves or ≤ 19mm in bicuspid non-fused aortic cusp (Fig. 5).
- The coaptation height (cH) or coaptation lenght (cL) is the distance of the cusp apposition in diastole by echocardiography. The normal range is 4-5mm.
- The lenght of the free margin of an aortic cusp is approximately 1.5 times the lenght of its base.
Mechanisms of aortic insufficiency
Repair-oriented classification of AI
During the last decades different techniques of aortic valve repair has been developed. However, the reproducibility of these techniques and adaption into daily clinical practice has been difficult. Therefore, El Khoury and collaborators published a repair-oriented classification of AI to guide surgical repair and improve clinical outcomes. El Khoury was inspired by Carpentier´s classification for mitral valve insufficiency and described the disease mechanism and repair techniques for AI accordingly (Fig. 6).
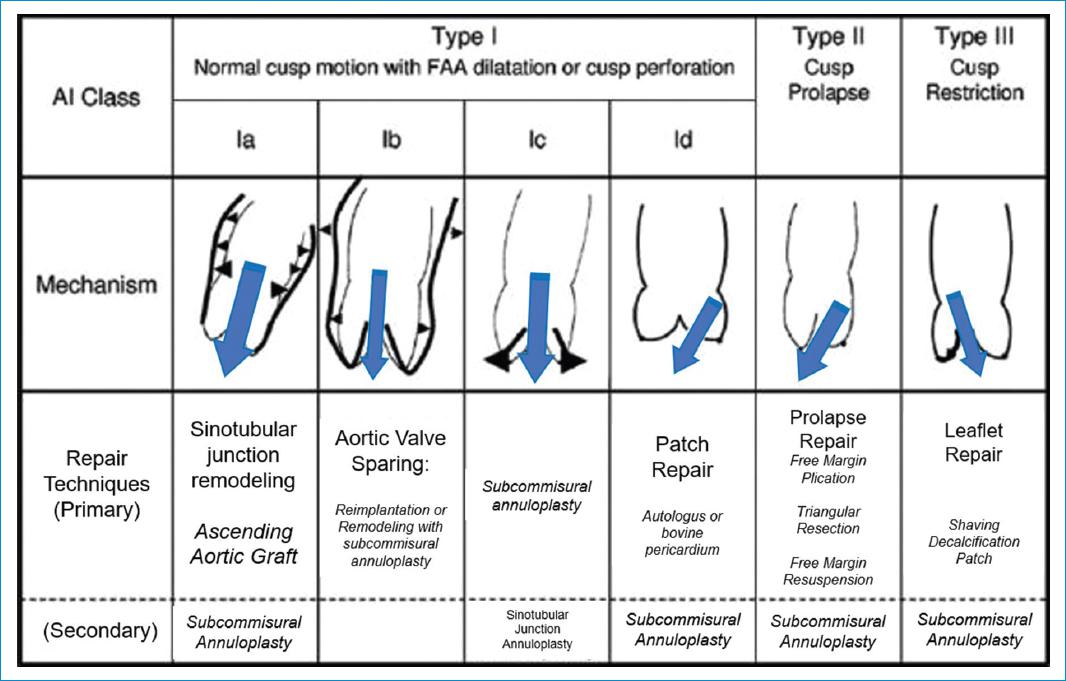
Figure 6 Repair-oriented functional classification of aortic insufficiency (AI) with description of disease mechanisms and repair techniques used. FAA, Functional aortic annulus; STJ, sinotubular junction; SCA, subcommissural annuloplasty. Adapted from: Boodhwani M et al.15
-
- Type I: AI associated with normal leaflet motion (largely due to lesions of the functional aortic annulus) or cusp perforation
- Type II: AI due to leaflet prolapse as a result of excessive cusp tissue or commissural disruption.
- Type III: AI due to leaflet restriction that can be found in bicuspid, degenerative or rheumatic valvular disease as a result of calcification, thickening and fibrosis of the aortic valve leaflets.
Patients can have multiple lesions that contribute to AI from different concomitant mechanisms, that makes necessary the use of several techniques in the same case.
Aortic valve repair techniques and outcomes
Sino-tubular junction remodeling
STJ Remodeling is the treatment for Type Ia AI, caused by a supracoronary ascending aorta aneurysm with concomitant dilation of the STJ (Fig 7).
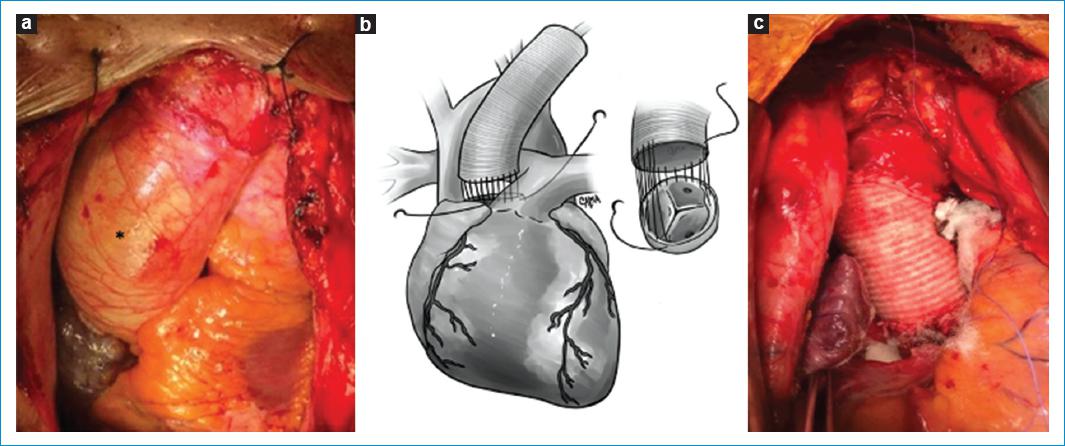
Figure 7 A: picture of an Ascending aorta aneurysm (*) with STJ dilation. B-C: ascending aorta replacement and STJ remodeling with a dacron tube graft. Technique description: The Ascending Aorta and the STJ (just above the commissures) are resected. The graft is sized with a valve sizer and the proximal anastomosis of the dacron tube graft is done with continued polypropylene suture. The dacron tube is cut at the appropriate length and the distal anastomosis is performed.
Aortic valve sparing: Reimplantation
Reimplantation of the aortic valve (RAV) technique is used in Type Ib AI, and was described in 1992 by David and Feindel. Nowadays it is known as the David Technique16 and described in Fig. 8.
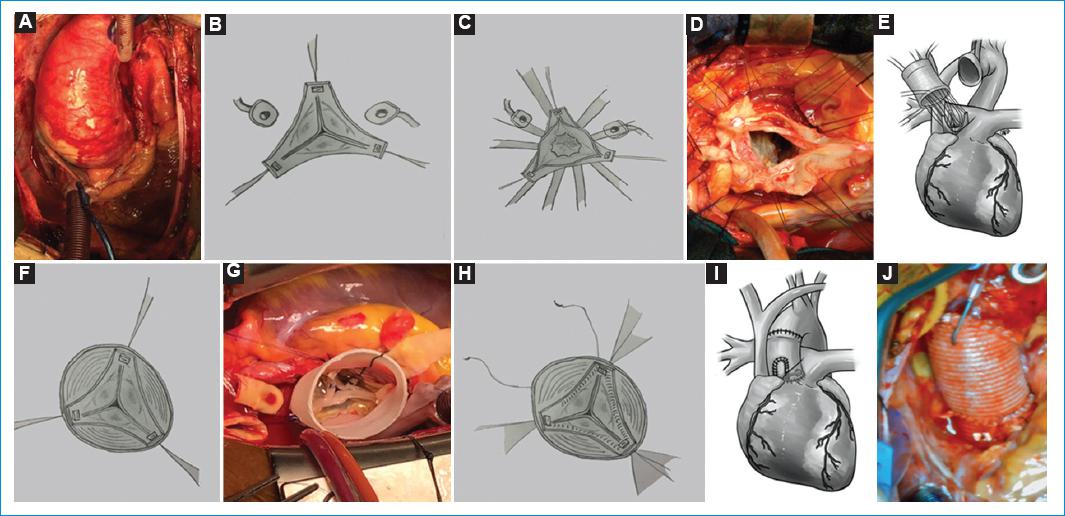
Figure 8 A: picture of Ascending aorta aneurysm with STJ and sinus of Valsalva dilatation. B: the aorta is resected to the root, excising the sinuses of Valsalva, and resecting the tissue to the level of the AA. The coronary buttons are excised. Sutures are positioned in every commissure to resuspend the leaflets. C-D: sutures are passed through the AVJ, several mm below the insertion of the leaflets to stabilize the AA. E: sutures are then passed through the bottom of the dacron tube graft and tied down. F-G: resuspension of the valve inside the dacron graft H. Hemostatic polypropylene continued suture is performed to suture the valve to the graft. I-J: coronary buttons are sutured to the graft and the anastomosis either to the aorta or to another dacron graft.
Tirone David´s group published the largest patient series including 333 young adults who underwent RAV between 1989 and 201217. They showed excellent results and stable aortic valve function at 20 years. Freedom from reoperation at 15 to 20 years was 96.9 - 1.3%. However, these data are showing a single-center experience from a high-volume expert center.
Aortic valve sparing: Remodeling
The remodeling technique was described in 1993 by Sarsam and Yacoub and can be used in type Ib AI. Nowadays it is known as the Yacoub technique18 (Fig. 9).
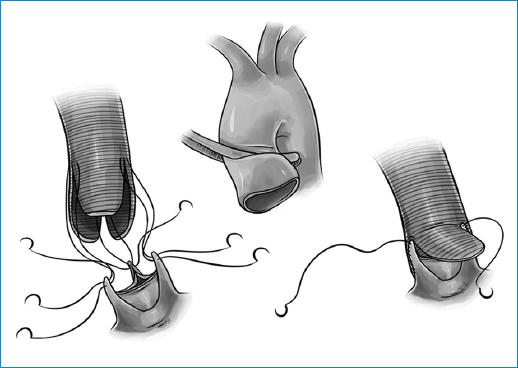
Figure 9 The aorta is resected to the root, excising the sinuses of Valsalva, and resecting the tissue to the level of the AA. The coronary buttons are excised and the tube is tailored creating 3 neo-sinuses. A polypropylene continued suture is performed to suture the tailored graft to the remaining aorta next to the leaflets. The coronary buttons are sutured to the graft and the distal anastomosis to the aorta is performed.
Although the early results after aortic valve sparing operation with remodeling were positive, the late results showed that a significant proportion of patients needed reoperation for recurrent AI19. This was most likely related to the lack of annular stabilization. Schäfers et al published their long-term experience with root remodeling in 2015. The retrospective analysis included 747 patients. The last 295 patients were also treated with a suture annuloplasty. Overall freedom from reoperation was 95% for tricuspid valves and 83% for bicuspid valves at 15 years. The strongest predictor for failure was an aortoventricular junction of 28mm or greater or the use of a pericardial patch as part of the cusp repair. Further developments and standardization of the remodeling root repair with a calibrated expansible aortic ring annuloplasty and cusp effective height assessment has shown to improve valve repair outcomes20. Lansac and colleagues published their results of 177 patients and showed that both factors reduce the risk for reoperation20.
Aortic valve sparing operations versus composite valve grafting
There are no randomized controlled trials available comparing both techniques. A recent meta-analysis included 26 adjusted and unadjusted observational studies comparing aortic valve sparing operations to composite valve grafting1. The analysis included 3.794 patients undergoing composite valve grafting and 2.424 patients undergoing aortic valve sparing procedures. Early mortality, myocardial infarction or thromboembolic complications were similar in both groups. Later mortality, late thromboembolism or stroke and bleeding risks were significantly lower in patients undergoing aortic valve sparing operations. Late durability was equivalent. Given these results aortic valve sparing operation should be considered in patients with favorable aortic valve morphology. Of note, most of the included studies were performed in expert centers for aortic valve sparing operations.
Annuloplasty
Annuloplasty aims to stabilize the annulus to prevent it from dilation in the long-term. This technique is associated to the remodeling procedure, in type Ic AI and in isolated leaflets procedures. Different techniques have been developed to achieve this goal including internal and external prosthetic rings and different suture techniques (Fig 10).
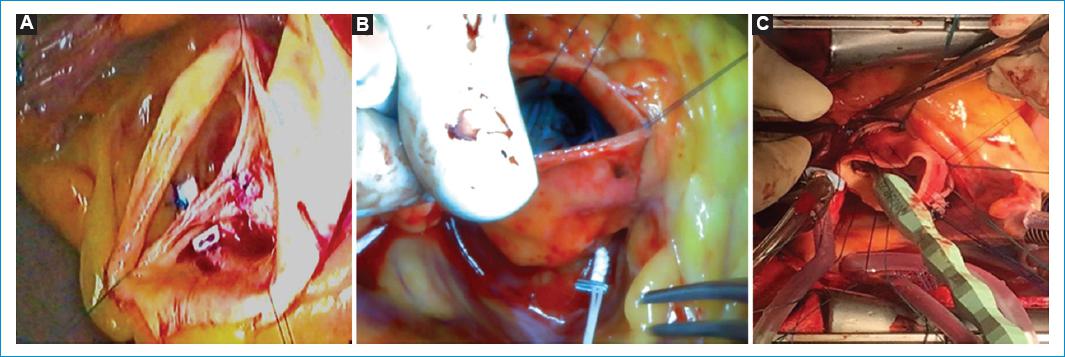
Figure 10 Aortic annuloplasty. A: subcommissural annuloplasty: With braided pledgeted sutures, a suture point is done from the aortic to the ventricular side, with the 2 needles, at the midcommissural height (except at the non-coronary/right coronary commissure, where it should be higher to avoid injuring the conduction tissue) and them tied down. B: external suture annuloplasty: Gore-Tex CV-0 suture is used, passing externaly through the nadirs of the three coronary sinus, starting from the right sinus below the ostium. Carefull care is needed to pass around the RC-NC commissure, deepening in the right ventricle and away from the membranous septum. A Hegar stem or a caliper of the desired size is use to tie down the knot in the nadir of the NC. C: external annuloplasty ring with a dacron ring. After deep dissection of the root, braided sutures are inserted at the AA in the same way that in the David´s technique. The ring is passed under the coronary ostia and between the AA sutures. The sutures are tie down carefully around a caliper or a Hegar stem of the desired size.
The outcomes of annuloplasty alone are difficult to interpret because procedures vary and numbers of studies are rather small. Schneider et al showed that both external and internal suture annuloplasty improved significantly repair stability in regurgitant bicuspid aortic valves. Freedom from reoperation after 5-years improved to 92.6% compared with 73.2% in patients treated with no annuloplasty21. Subcommissural annuloplasty was frequently associated with reoperation and aortic regurgitation greater than grade II22,23. This method was also associated with increased postoperative transvalvular gradients of more than 25mmHg24. Rigid or flexible rings were also studied. AVIATOR, a multicenter prospective study from France, showed that an external, flexible ring annuloplasty combined with remodeling using a graft with sinuses provided in 177 patients freedom from reoperation of 89.5% at 7 years. In bicuspid valves the rate was 100%25. Interestingly, the authors insisted that double annuloplasty by adding an external ring at the STJ level reduced the risk for relevant aortic regurgitation20. As insights into aortic valve repair have deepened during the last years, annuloplasty has emerged to play an important role. However, the procedure is mainly performed in highly experienced centers and needs further investigations to determine which method can become the standard option.
Leaflet repair: Free margin plication and resuspension
Prolapse of the leaflets can be corrected by free margin plication (Fig. 11) or free margin resuspension (Fig. 12).
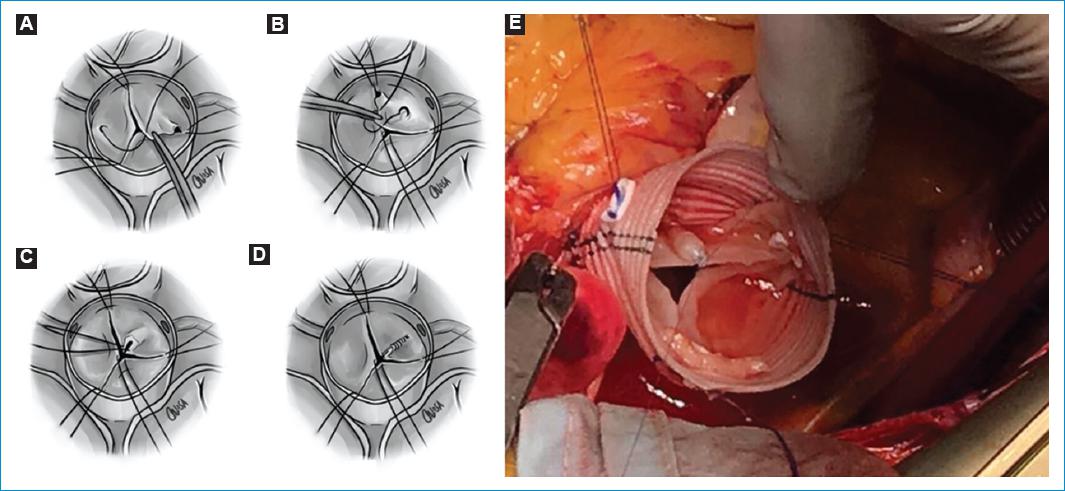
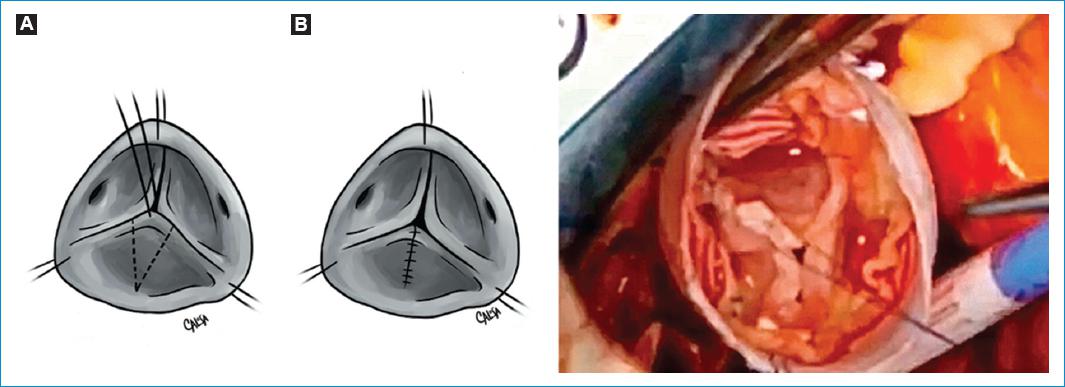
Figure 11 Free margin plication. A-D: a polypropylene suture is passed through a central point in which all three leaflets are joined. Then, the central stitch is gently pulled to each commissure, checking that the edges are parallel. If there is a longer one, it will be the one prolapsing. The excess of tissue is plicate with a polypropylene suture, running towards the body of the cusp, leaving the excess of tissue on the aortic side to restore the geometry of the leaflets. E: free margin plication of the Right coronary cusp.
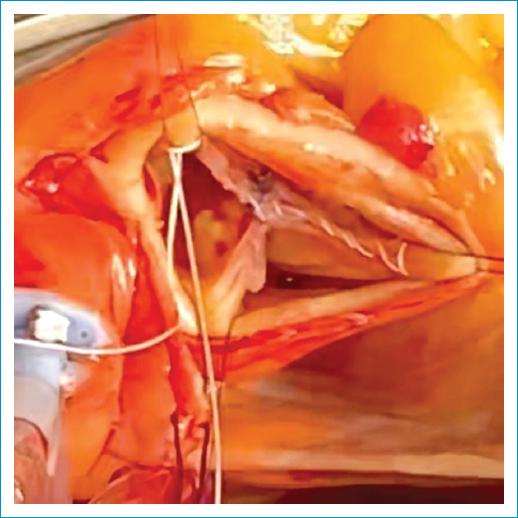
Figure 12 Free margin resuspension of the Right coronary cusp: Excess length of the free margin is corrected with a polytetrafluoroethylene (PTFE) suture. The suture is passed from commissure to commissure in a running fashion over the length of the free margin. The length is reduced applying gentle traction in the suture and them the suture is tied down.
Leaflet repair: Triangular resection
Triangular resection of a cusp is used to remove the excess of tissue (Fig. 13).
Leaflet repair: Patch repair and augmentation
The patch repair technique is used to cover defects that involve the body of the valve caused by endocarditis, trauma or iatrogenic (Fig 14).
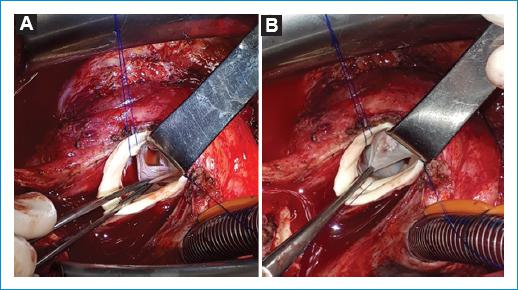
Figure 14 A: traumatic perforation of the right coronary cusp. B: a pericardium (autologous or heterologous) patch is tailored according to the shape and size of the defect, adding a 2mm margin for suturing. The patch is suture with polypropylene closing the defect.
A pericardial patch can also be used to augment tissue to a small or retracted leaflet or after resection to restore the leaflet (Fig 15).
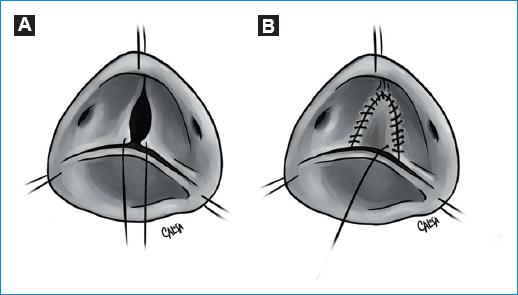
Figure 15 A-B: patch augmentation. Severely restricted or calcified raphes can be resected and then restored with a pericardial patch. This is also possible in cases of small cusps.
The outcomes of leaflet repair techniques are difficult to compare, because in daily practice many patients are treated with more than one technique according to the need. The Brussels group published a large serie including 146 patients26. Almost half of them underwent more than one technique. Resuspension was performed in 109 (75%), triangular resection/suture in 51 (35%), central plication in 47 (32%), and pericardial patch in 10 cases (7%). After 4 years of follow-up there was no difference in the freedom from recurrence of regurgitation between central plication vs. resuspension vs. combined procedures (95 ± 8%, 83 ± 18%, and 100%, respectively (p = 0.37)). However, rates were significantly worse for triangular resection versus pericardial patch unless resuspension was performed simultaneously.
Leaflet repair using pericardial patches has shown mixed results. Data from the homburg group showed reasonable mid- and long-term durability in tricuspid aortic valve repair but poor results in bicuspid aortic valve repair regardless of cusp pathology and repair technique27.
In general, experienced centers for aortic valve repair were able to publish remarkable results. The homburg group reported 640 cases of aortic valve repair with 5- and 10-year rates of freedom from reoperation of 88% and 81% for bicuspid and 97% and 93% for tricuspid valves, respectively. The Brussels group reported 475 cases of aortic valve repair with a 10-year survival rate of 73.5 ± 5%, and a freedom from valve-related death of 90 ± 3%28. The freedom from significant AI was 84 ± 3%, freedom from aortic valve reoperation was 86 ± 3%, and freedom from aortic valve replacement was 90 ± 3%. The Mayo group reported 331 cases of aortic valve repair with 5- and 10-year survival rates of 91% and 81%, respectively29.
Special considerations and a new classification in bicuspid aortic valve repair
Patients with regurgitant bicuspid aortic valves often present with dilatation of the aortic annulus, aortic root or ascending aorta. Therefore, almost always multiple mechanisms can be identified to cause AI. Historically, the classification system of Sievers and Schmidtke was used to divide bicuspid aortic valves into two groups as mentioned above30. Recently, de Kerchove and colleagues developed a more anatomical and repair-oriented classification for bicuspid aortic valves to guide surgical repair13 (Fig.16).
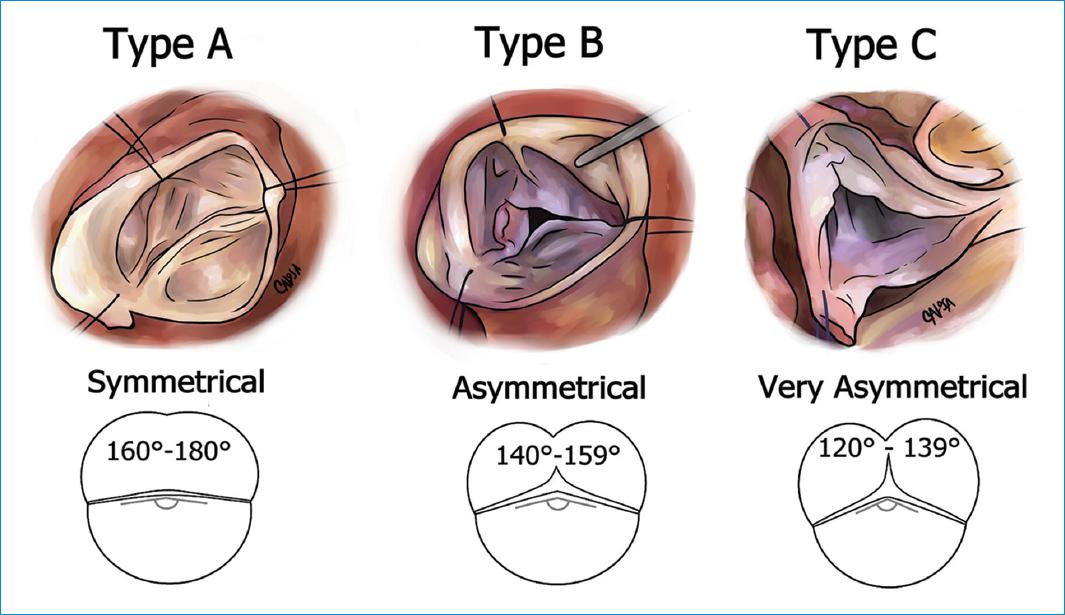
Figure 16 Schematic echocardiographic and intraoperative illustrations of the 3 groups of phenotypes of the repair-oriented bicuspid aortic valve classification.
The study population included 178 consecutive patients with bicuspid aortic valves operated for AI or aortic dilatation. They found that bicuspid aortic valves phenotypes followed a continuous spectrum that extends from symmetrical to very asymmetrical bicuspid aortic valves and divided patients into 3 phenotypic groups according to their commissural orientation. Patients with a commissural orientation from 180°-160° where classified as "Type A", patients with a commissural orientation from 159°-140° as "Type B" an patients with a commissural orientation from 139°-120° as "Type C". The commissural orientation correlated positively with the length of the raphe fusion and negatively with the height of the nonfunctional commissure13. Aortic valve replacement and residual AI were significantly more frequent in Type C. According to the new classification system the authors recommended specific repair techniques which were used successfully for each phenotype in Brussel and in Homburg. In patients with symmetrical phenotypes, cusp prolapse can be treated in standard fashion using central plications. In patients with an asymmetrical phenotypes the commissural orientation should be increased towards 180°. This technique allows primary closure of the non-fused segment of the fused cusp while preserving its mobility and avoiding patch extension. To achieve this goal valve-sparing techniques are useful which can make the valve symmetrical or by the sinus plication stitch, which increases the commissural orientation. Patients with the very asymmetrical phenotypes are probably best treated like a tri-leaflet valve by plication of one or two of the residual cusp components of the fused cusp or by creation of a functional commissure at the place of the raphe. Very asymmetrical phenotypes can also be transformed into more symmetrical bi-leaflet valves using the techniques described for asymmetrical phenotypes. The new classification system from de Kerchove and colleagues includes the entire spectrum of bicuspid aortic valve disease an uses measurable valve parameters and may be able to predict valve repair techniques. However, it needs further validation with regards to long-term outcomes in the future.
How to set up your aortic valve repair program
Heart valve team
Aortic valve repair procedures are complex, have progressed slowly during the last decades and are still only performed in highly specialized centers. To set up your own aortic valve repair program we believe that the concept of a "Heart Valve Team" is important to be able to discuss all available treatment options tailored to the disease of the patients and therefore optimize patient selection, procedural performance and follow-up care. The Heart Valve Team includes cardiac surgeons, cardiologist specialized in imaging and interventions, cardiac anesthetist and intensive care specialists. All patients must be evaluated preliminary by this team, in order to stablish the suitability of the repair. A Transesophageal echocardiogram performed by a cardiologist specialized in imaging, is mandatory and must report the mechanism of the regurgitation, presence of prolapse, thickness of the leaflets, different anatomical measurements (fig. 4), and identified difficult escenarios for aortic valve repair, such as leaflet retractions, calcification, symmetry in bicuspids, among others. Also an angio-CT must always be performed to have an objective of measure the diameters of the aorta. If the root is dilated, the study of all the aorta is required. We strongly believe, that open-minded discussions in an interdisciplinary Heart Valve Team allows to facilitate patient- centered, innovative and evidence-based care with optimal outcomes. Establishing a format of such a Heart Valve Team will be essential to start your successful program.
Training
Proper training in aortic surgery with a large experience in aortic valve surgery is mandatory before beginning with an aortic valve repair program. Look out for opportunities to get in contact with the pioneers of the field and benefit from their experience. Visit high volume expert centers to observe their surgical performances and department structures and try to benefit and gain knowledge as much as possible.
First cases- the key for success
Once you start your program begin with easy cases, progress slowly and ask for proctoring if needed. First cases are of utmost importance to become accepted by referring physicians, cardiologists and patients.
Facilities and reimbursement
There are multiple healthcare economic models in different countries, however you have to make your own adapted economic analysis having in mind your facilities, resources and costs for your institution and your patients. This needs to be discussed well in advance before you start your program.
Outcome research and quality control
For quality control it is important to maintain a database to report and track your results. Regular follow-ups in your valve clinic are highly recommended to prove short- and long term durability of your procedures and to be able to improve over time. In our center we stablished a postoperative follow-up schedule at 1, 6, 12 months and them annualy after the procedure, with Transthoracic echocardiogram. This also allows you to contribute to national and international registries. If you are able to show and publish your data, your program is more likely to be accepted by referring physicians and cardiologists.
Conclusion
Aortic valve repair has evolved during the last decades, but it is still mainly reserved to high- volume and specialized centers. Outcome data are promising in terms of durability and survival. However, long-term studies comparing different valve repair strategies are missing and of utmost interest for the future. We believe that beside high- quality training, facilities and resources an interdisciplinary, open minded and innovative heart valve team may help to implement a successful aortic valve repair program at your clinic.