Introduction
Atherosclerotic coronary disease is the main mechanism to develop an acute myocardial infarction. Other mechanisms are non-atherosclerotic coronary disease and hypercoagulability states, like gene mutations associated with hereditary thrombophilia. Several studies suggest that gene mutations from thrombophilia may constitute a significant risk factor for coronary disease, especially in young patients with normal coronaries and non significant lesions1-6. The previous studies show that this combination, of common risk factors and thrombophilia, is associated with a high risk of death and recurrence of acute myocardial infarction within 2 years after the first episode1,2.
Therefore, it is presented a clinical case consistent with several common risk factors for coronary heart disease with multiple thrombi in different coronary arteries, even in a non-culprit coronary vessel during acute myocardial infarction. Despite the presence of conventional coronary risk factors, it was not enough to explain spontaneous thrombi appearing during primary percutaneous coronary intervention in a vessel not related to the infarction and without any angiographic lesion to blame at this site. Therefore, laboratory assessment to search for primary and secondary risks factors for thrombosis was justified with the finding of two gene mutations for thrombophilia.
Clinical case
A 42-year-old male patient arrived at the emergency room due to an acute myocardial infarction. He came to the emergency room due to chest pain, oppressive, retrosternal, radiating to the left arm, at rest, and mild-to-moderate intensity. He denied having dyspnea, cold sweats, nausea, vomits, or other symptoms. He also denied arterial hypertension, diabetes, dyslipidemia, or thyroid disease. He did not have family history of coronary artery disease. He is a former smoker, not alcohol consumer, neither of illicit drugs. At physical examination, he was obese (BMI = 35 kg/m2), the pulmonary examination was normal, no signs of breathing difficulty, normal vital signs, blood pressure 140/87 mmHg, heart beat 95/min, respiratory frequency of 20/min, body temperature of 36.5°C, and oxygen saturation of 96% in room air.
A 12-lead conventional electrocardiogram (ECG) showed an ST elevation with upward convexity of 4 mm from V1 to V4 and of 1 mm at V5 and V6 (Fig. 1A). Further conventional ECG showed T negative waves from V1 to V6 and D1 to aVL (Fig. 1B). Chest X-ray showed no cardiomegaly or signs of pulmonary edema. On laboratory tests, assessment showed that cardiac enzymes were positive (troponin I = 8.4 ng/dl, normal range < 1 ng/dl). He had a high cholesterol test of 327 mg/dl (normal range < 200 mg/dl), high density lipoprotein cholesterol 25 mg/dl (normal range > 40 mg/dl), low-density lipoproteins cholesterol 244 mg/dl (normal range < 100 mg/dl), and very low-density lipoprotein cholesterol 58 mg/dl (normal range < 40 mg/dl). He had normal levels of fasting glucose, hemoglobin A1C, uric acid, creatinine, and homocysteine. At trans-thoracic echocardiography, the diameters of the left ventricle at diastole and systole were 56 mm and 45 mm, respectively. The ejection fraction by Simpson method was 44% with septal hypokinesia (medium-apical) and apical hypokinesia. No thrombus was found in the left ventricle. The patient was started on treatment with dual anti-platelet therapy with aspirin and clopidogrel, anticoagulation with low molecular weight heparin, atorvastatin, angiotensin convertor enzyme inhibitor, betablocker, and aldosterone inhibitor.
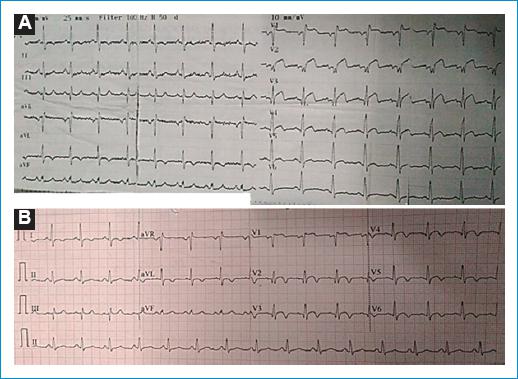
Figure 1 A: electrocardiogram (ECG) at the emergency room with ST segment elevation with an upward convexity of 4 mm from V1 to V4, 1 mm at V5-V6. B: ECG following angioplasty shows near normal ST segment, with symmetric T negative waves from V1-V6, D1-aVL.
A coronary angiography was performed, showing a total occlusion of the left anterior descending coronary artery in the middle segment which represents the culprit lesion of the infarct. There were other occlusions such as at a proximal level in the second marginal branch of the circumflex artery and a severe lesion in the distal segment of the right coronary artery (Fig. 2). The culprit lesion of the infarction was treated with primary angioplasty, successfully dilating the artery with a balloon, with a resultant thrombolysis in myocardial infarction (TIMI) 2 flow in the middle and distal segments of the vessel. Just before the stent placement, there were spontaneous thrombi appearing at the middle segment of the circumflex artery, at an area that did not present previously occlusive atherosclerotic lesions (Fig 3A). An abciximab infusion was started as continuous infusion for 24 h. He also received nitroglycerin infusion for 36 h due to a mild elevation of blood pressure. After 48 h, a new coronary angiography showed a reocclusion at the middle segment of the left anterior descending coronary artery, with a decrease in the thrombus burden in the circumflex artery. Dilation with a balloon and placement of a zotarolimus-eluting stent resulted in a TIMI 3 flow through the artery (Fig. 3B). Patient evolved without complications during the rest of the hospital stay. A new echocardiography showed a left ventricle ejection fraction of 47% (Simpson method).
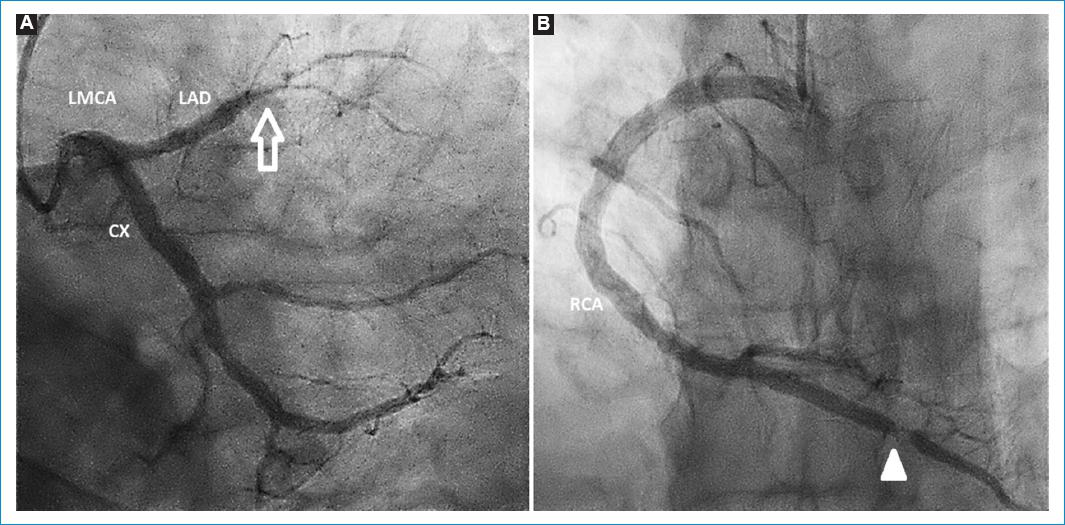
Figure 2 Coronary angiography. A: complete occlusion of the left anterior descending coronary artery in its middle third (arrow). B: severe occlusion at the last third of right coronary artery (arrow head).
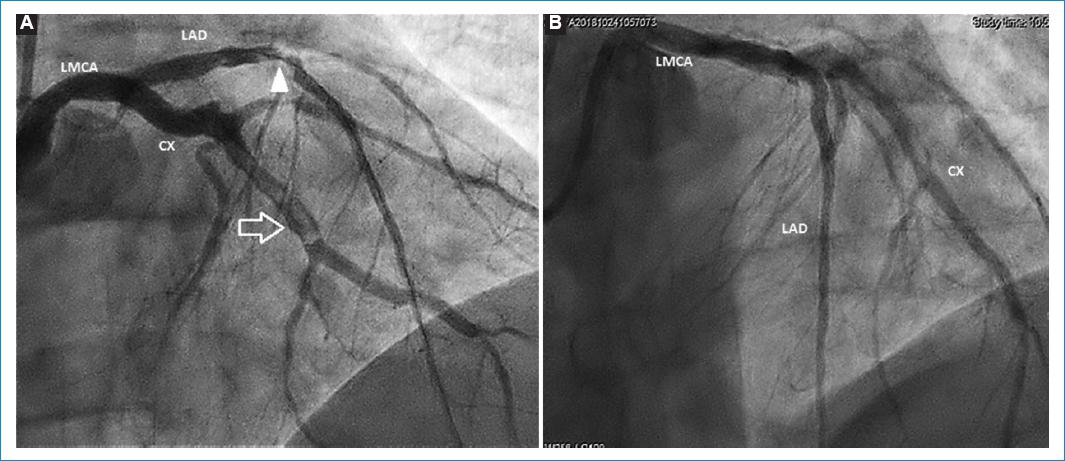
Figure 3 A: primary angioplasty of the left anterior descending artery shows a severe lesion at the junction of the proximal and middle third (arrow head). During the procedure, spontaneous cloths were found in the middle third of circumflex artery (arrow). B: 48 h after the angioplasty, after IIb/IIIa glycoprotein inhibitors, decreased thrombus burden in the circumflex artery.
Due to the spontaneous thrombi found at the coronary artery that did not relate to the myocardial infarction territory, without any atherosclerotic plaques to blame in a young patient, it was mandatory to perform a laboratory assessment for prothrombotic states diseases. The results were negative for antiphospholipid antibodies (lupus anticoagulant = 0.83, NV < 1.2; anticardiolipin IgG = 1.4, NV < 10 GPL-U/ml; anticardiolipin IgM = 0.4, NV < 7 MPL-U/ml; anti-beta 2 glycoprotein I IgG = 1.4, NV < 5 U/ml; and anti-beta 2 glycoprotein IgM = 0.4, NV < 5 U/ml). Other genetic mutations associated with thrombophilia resulted negative, such as mutations for factor V (Leiden, Cambridge, Liverpool, and Hong Kong), for prothrombin mutation (M 20210A), negative for MTHFR 677T mutation (methylene tetrahydropholate reductase), but positive for mutations at A1298C from the MTHFR gene heterozygote genotype and plasminogen activator inhibitor-1 (PAI-1).
Therefore, this patient was considered at high risk for trombophilia and thrombosis with two genetic mutations. An anticoagulant treatment was installed with Vitamin K antagonists for a goal of international normalized ratio (INR) 2 to 3 as a secondary prevention. Furthermore, long-term dual antiplatelet therapy was started due to stent implantation, with a low risk of bleeding for triple therapy (Precise-DAPT score 8; HAS-BLED score 1). The patient was discharged in well condition and comes to consultation regularly at the cardiology department, without myocardial ischemic symptoms or other thrombotic complications, no bleeding as well (Figs. 1-3).
Discussion
This was a young patient with several common risk factors for coronary artery disease but also with primary risk factors such as thrombophilia with two genetic mutations, in this case, gen mutations at A1298C from the MTHFR and PAI-1. The previous studies have shown that this combination of common risk factors for atherosclerosis and thrombophilia is associated with a high risk of death and recurrence of acute myocardial infarction within 2 years after the first episode1,2. Furthermore, in the absence of classic risk factors or without significant occlusions, hereditary thrombophilia predispose to the development of the first myocardial infarction3-5.
Studies have shown that polymorphism of mutations at A1298C from MTHFR gene predisposes to coronary artery disease in some patients6. The prothrombotic mechanism described is the lower activity of 5,10-methylene tetrahydrofolate reductase enzyme, which catalyzes methylation from homocysteine to methionine, producing high levels of homocysteine. There is a strong relationship between high levels of homocysteine and cardiovascular disease7-9. The mechanisms include lower production of nitric oxide from endothelium, proliferation of smooth muscle fibers, endothelium dysfunction, and effects on platelets and coagulation8. The patient in the present case had normal levels of homocysteine. A decreased function of MTHFR enzyme activity can lead to the high levels of homocysteine. This does not necessarily happen, especially in countries like USA, where food is enriched with folate. In that part of the world, the mutation in the absence of the high levels of homocysteine does not constitute a risk factor for heart disease. Even with the high levels, the treatment to reduce it, do not improve the risk of recurrent events7. The Thrombosis Interest Group of Canada do not recommend testing serum levels of homocysteine in samples of patients with heart disease and venous thromboembolism. They do not recommend testing for MTHFR mutation as part of the routine examination for thrombophilia9. However, such scenario does not apply to our country population due to ethnic and dietary differences. A regional study in Latin-American population showed high prevalence of MTHFR gene mutation and its association with hyper-homocysteine and heart disease development10. In patients with reduced MTHFR enzyme activity and consequently hyper-homocysteinemia, daily low-dose folic acid supplementation can reduce and often normalize their homocysteine levels. However, this measure has not been shown to improve clinical outcomes11. Clinical trials and meta-analyzes have shown that lowering homocysteine levels with Vitamin B6, B9 (folic acid), and B12 supplementation did not reduce cardiovascular complications even in patients with elevated baseline levels12. At present, based on available data, treatment of hyper-homocysteinemia is not recommended in patients with cardiovascular disease.
Regarding plasminogen activator inhibitor-1 (PAI-1), this is an important inhibitor of the fibrinolytic system and it is produced at the vascular endothelium and is also present in platelets13. Suppression of fibrinolysis happens with the high levels of PAI-1 and with high levels of factor VII, fibrinogen, and von Willebrand factor, a myocardial infarction can be the result of this combination14. Besides, the high levels of plasminogen activator (t-PA) and dimers-D increase the risk of myocardial infarction15. The high levels of PAI-1 were associated with vascular inflammation, atherosclerosis, and metabolic syndrome16. High activity of PAI-1 has been detected in atherosclerosis of patients with obesity and Type 2 diabetes mellitus. The high levels of PAI-1 were described related to the development of first myocardial infarction17 and with progression of coronary syndromes and coronary artery disease in patients with the previous myocardial infarction.
Although the present patient described also had several common risk factors for coronary heart disease, it was not enough to explain spontaneous thrombi appearing during the procedure in a vessel not related to the infarction and without any angiographic lesion previously. Therefore, laboratory assessment to search for primary and secondary risks factors for thrombosis was justified, with positive findings in more than one gene mutation. As for therapeutics, dual anti-platelet therapy alone cannot prevent new thrombotic events due to two mechanisms involved, thus triple therapy should be considered with long-term anticoagulation to prevent new episodes.














