Introduction
Access to new experimental techniques based on assertive methodologies to the exploration of the biological entity has allowed researchers to achieve a better knowledge in the area of biomedicine, both at the structural and functional level of the different organs and tissues. In addition, it has been possible to reorient pathologies toward more specific management, with which the health conditions of the population have been significantly impacted.
The combination of complementary techniques and methods, contributed by the basic sciences to the context of clinical application research, have led to versatile in vitro experimental platforms, as is the case of cell cultures, a promising environment for research in the cardiovascular area1. This research field then fosters the implementation of cell and tissue banks through the development of platforms for tests framed in different technical-scientific approaches which allow the modeling of those natural conditions of the tissue or organic structure under study.
By means of in vitro experimental platforms in the context of cell cultures, it is possible to study aspects ranging from normal cardiac physiology to different pathologies that affect the cardiocirculatory system, thus allowing the response to be studied after the intervention with multiple pharmacological therapies, physical procedures or the use of new materials, among others2.
Consequently, a new field of scientific environment is generated, where professionals from various disciplines work together and which involves varied laboratory practices and techniques aimed at modeling scenarios to study the behavior of living tissue. In this context, cell cultures serve as a model before experimental work on laboratory animals and human subjects which makes it a valuable tool for broad models of study in the area of cardiology.
Cell cultures
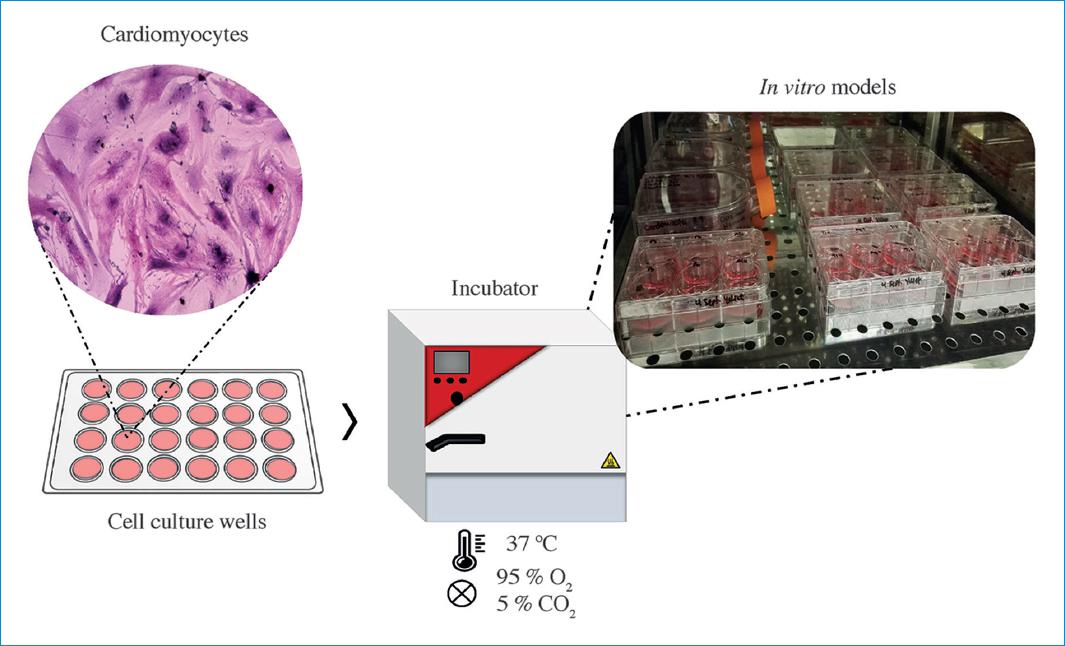
The cell culture technique consists of the isolation of cells in an artificial environment conducive to their growth and development, so that their expression and functionality are equivalent to the tissue in the organism. By controlling cell to cell and extracellular cell-matrix interactions by means of two-dimensional and three-dimensional models composed of both isotropic and anisotropic structures, it is possible to obtain controlled microenvironments to evaluate physiological responses to external agent stimuli, as is the case of pharmacokinetic, cytotoxic, mutagenic, and inflammatory responses due to the exposure to medicines and biomaterials, among others3.
Unlike cells confined in their natural environment within the organism, isolated cells in culture platforms may be circumscribed in a defined environment determined by conditions imposed for the study. This differentiates them from the former, the native cells, which are subjected to disturbances and other interrelations with their natural biological environment (Table 1).
Table 1 Differences between native cells of the organism and cultured cells
| Native cells |
| –They are subjected to natural regulatory conditions of the organism in the human body. |
| –Their behavior is evidenced by their interrelation with other tissues. |
| Cultured cells |
| –They are under controlled conditions in confined environments in the laboratory. |
| –Their specific behavior is evidenced by the cells under study themselves. |
In vitro cardiac cell models are becoming a generic practice that addresses issues ranging from the availability of specific nutritional media for the cells to the replication of physical conditions conducive to their development, such as pressure and temperature environments and gas concentrations, among others4. In addition, it involves a series of instrumental, biochemical, and molecular techniques that provide broad access to the multiplicity of functional variables and parameters which are to be studied within the cell cultures.
In the area of the cardiovascular medicine, cell culture techniques may entail cells, such as cardiomyocytes and cardiac fibroblasts, as well as endothelial cells. They all demand careful management of conditions for the vital and functional conservation of the cells, in addition to the conditions related to the set of requirements such as sterility, asepsis and biosafety zones in the case of tissue banks5.
Cell lines
Generically, there may be two types of cell cultures: established cell lines or primary tissue lines. Established cell lines are immortalized cell cultures whose cells derive from a controlled selection process and maintain their lineage through a replication mechanism. Primary tissue cells are those cultures whose cells derive directly from a tissue or an organ so they may contain many types of non-strictly differentiated cells. In this context, while established cell lines allow an extensive number of replications, primary tissue lines have a very limited number of functional replications.
Since cultures may be of both lineages, their availability and acquisition are different. While some may be obtained as commercial lines, others must be extracted from explants and enzymatic digestions by means of techniques that allow suitable growth in monolayers.
Established lines
There are many types of established cell lines. They depend on the study models to be implemented according to the pathologies or physiological phenomena under analysis. As a consequence, if an evaluation of electrophysiological responses is required, cells that can be activated through electrical stimuli or expressing cell automatism are needed6. In turn, if an evaluation of contractility is required, cells that account for the shortening and relaxation phenomenon of myofibrils are required. To evaluate cytotoxic, mutagenic and inflammatory phenomena, an integral response from the genomic, proteomic and metabolic pathways, among others, is required7. In sum, a cell line cannot be used generically for any type of study.
Among the types of cells most frequently referred to in any cardiovascular research are: atrial and ventricular cardiomyocytes, cardiac fibroblasts and endothelial cells, of which there are several established cell lines (Fig. 1).
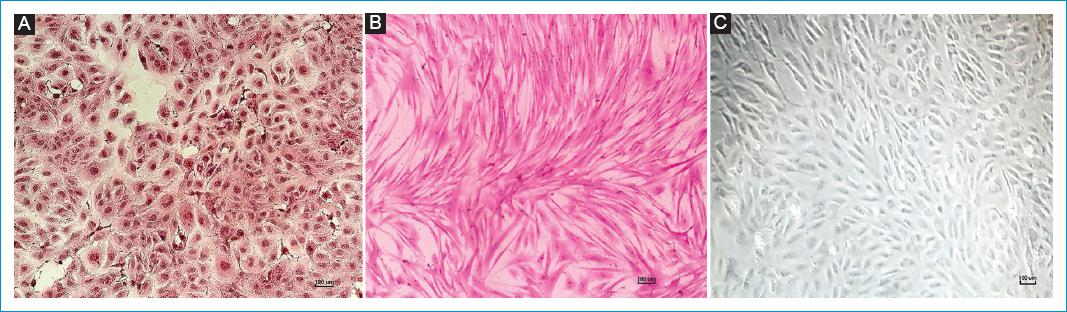
Figure 1 Cardiac cells culture with hematoxylin and eosin staining, A: RL-14 human cardiomyocytes. B: BALB/c mouse primary cardiac fibroblasts. C: human endothelial cells-HUVEC. Scale bar: 100 μm.Source: Cardiovascular Dynamics Group, Universidad Pontificia Bolivariana.
Human fetal ventricular cardiomyocytes, SV- 40 - RL14
They are immortalized cells derived from isolated post-mitotic heart primary cultures which are transformed by the monoclonal antibody SV-40. These cardiomyocytes express the b-myosin heavy chain, the connexin-43, and the proteins that allow gap junctions. The presence of intercellular junctions and the main protein of cardiac specific junction, connexin-43, will allow the cells to form a cellular syncytium8.
HL-1 cardiomyocytes
They are cells derived from atrial tumors of a female adult C57BLy6J mouse. They reproduce regularly and they are stable from generation to generation. These cardiomyocytes grow as monolayers, which keep the ability to contract since they have only one central nucleus surrounded by contractile myofibrils arranged in a circular fashion with myosin and desmin filaments9.
Human cardiac fibroblasts- NHCF V
They are cells isolated from the ventricular tissue of a healthy adult heart. These fibroblasts express a 90% of type-1 collagen and grow as a monolayer10.
Human endothelial cells – HUVEC
They are cells isolated from human umbilical cord veins which constitute a semi-permeable layer, termed the endothelial layer. They express all the activity of the endothelium, both endocrine and of cellular reactivity11.
Among other cells are the pluripotent stem cells12, which are categorized into cardiomyocytes and cardiac fibroblasts to be employed in immunohistochemical tests13, pharmacokinetics and electrophysiology evaluation14.
Primary tissue lines
They are cellular lines derived directly from a tissue or an organ, by means of explant techniques and the dissection of tissue thus obtaining fragments with growth potential in an in vitro environment15. Successful growth of these cells depends on the transport media of the organ or tissue, resection time, dissection quality, substrates fixation, culture method used, and the subsequent care for their maintenance16.
Finally, the type of cells obtained from the explanted organ, heart, or blood vessel depends on the dissected area since they may contain numerous types of undifferentiated cells in addition to the desired cells. In that sense, the cells functionality relates to whether such samples were taken from the auricles, the ventricles, the interventricular septum, or the valve apparatus, in such a way that they can include junction tissue, cardiac conduction bundles, autonomic nodes, or contractile fibers (Fig. 2). Otherwise, if samples are taken from the inner layer, the tunica media or the tunica adventitia of a blood vessel, they may include the endothelium, smooth muscle bundles or vascular fibroblasts.
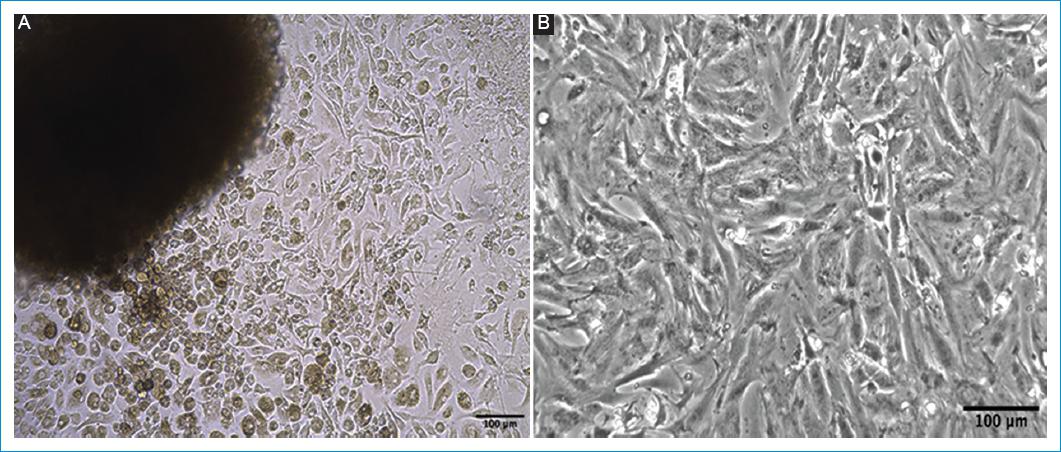
Figure 2 Isolation of primary cardiac cells. A: explant of the heart right ventricle. B: enzymatic digestion, of neonatal hearts of a 3-days old BALB/c mouse. Scale bar: 100 μm.Source: Cardiovascular Dynamics Group, Universidad Pontificia Bolivariana.
Media for the maintenance of cell cultures
Working with cellular cultures involves complying with different technical requirements to maintain their vital and functional conditions intact, which include elements related to the culture bank, thus allowing researchers to model the natural conditions of the tissue or organic structure under study. Consequently, careful handling of nutritional media to satisfy the demands of the tissue as well as of the physical conditions to protect its integrity is required. In addition, aseptic and biosafe areas to protect the samples from any type of cross contamination are required (Fig. 3).
Nutritional media
The availability of multiple specific culture media for the different lines of vascular or cardiac prototype cells is related to the high fragility of the environment for each type of cell to keep their vital functions and their homeostasis within their environment, from which their structural and functional integrity is derived.
Energetic, ionic, amino acid, replication, reproduction and structure synthesis requirements demand different nutrients, oxygen, pH conditions, and other conditions that ensure the bioavailability of the substrates required in different reactions and cell metabolic processes17. These requirements change according to the cell lines and study models to be implemented, whether to evaluate electrophysiological responses, contractility or to evaluate cytotoxic, mutagenic, genotoxic, or inflammatory phenomena18.
These nutritional media contain electrolytes, glucose, amino acids, vitamins, and other active oligo elements, through homogenized and balanced mixtures, in aqueous environments with defined osmotic pressures.
Physical media
Likewise, the replication of physical conditions such as pressures and temperatures suitable for their viability and functionality is required. These conditions are met by using different chambers or incubators that have automatized controls for such conditions. Similarly, a mixed environment of O2 and CO2 is used to ensure a suitable environment for cellular respiration19.
Relevance of the cell modeling technique
Various factors, ranging from ethical aspects, which limit the conduction of studies on human subjects or animals, to technical ones, which limit access to organic systems, open the door for new research techniques such as culture cells. In fact, these promising research environments, properly controlled and monitored, make it possible to conduct a large number of cardiovascular research studies. In these models, there are the evaluation of pharmacological agents, three-dimensional structures, biocompatibility of bioactive agents, and tissue regeneration processes20.
Given that there are multiple variables being manipulated which may simultaneously influence the human organism environment, it is necessary to identify and isolate those which actually disrupt the responses of the system under study, and to take into account only those variables of interest which need to be manipulated as part of the research work. This is possible through culture cell studies. Indeed, this experimental platform overcomes limitations associated to other traditional study systems, making it possible to explore different pathologies as well as diagnostic or therapeutic means in the cardiovascular system more effectively21. On the other hand, in vitro models of cardiac cells allow determining cell-cell interaction mechanisms, adhesion, proliferation, maturation, and cell differentiation processes, when interacting with external agents to propose our therapeutic strategies22.
Scenarios engaged in this kind of research resource
The implementation of work environments using culture cells requires interdisciplinary professional participation, considering the biological, biophysical and biochemical aspects involved which is part of physiological as well as physiopathological processes. In this context, there is a need for the cooperative work of experts, who include biologists, biophysicists, chemists, biomedical engineers and cardiologists, so that they can jointly determine the study model and assure interoperability in relation to aspects including cell culture and the elements and devices used to collect, analyze and interpret data23.
For the basic and biomedical areas, this need is starting to pose the challenge of approximating laboratory models to clinical arguments; and for cardiologists, the challenge of becoming familiar with new experimental fields already adopted by basic sciences long ago.
Lines of work arising from these platforms
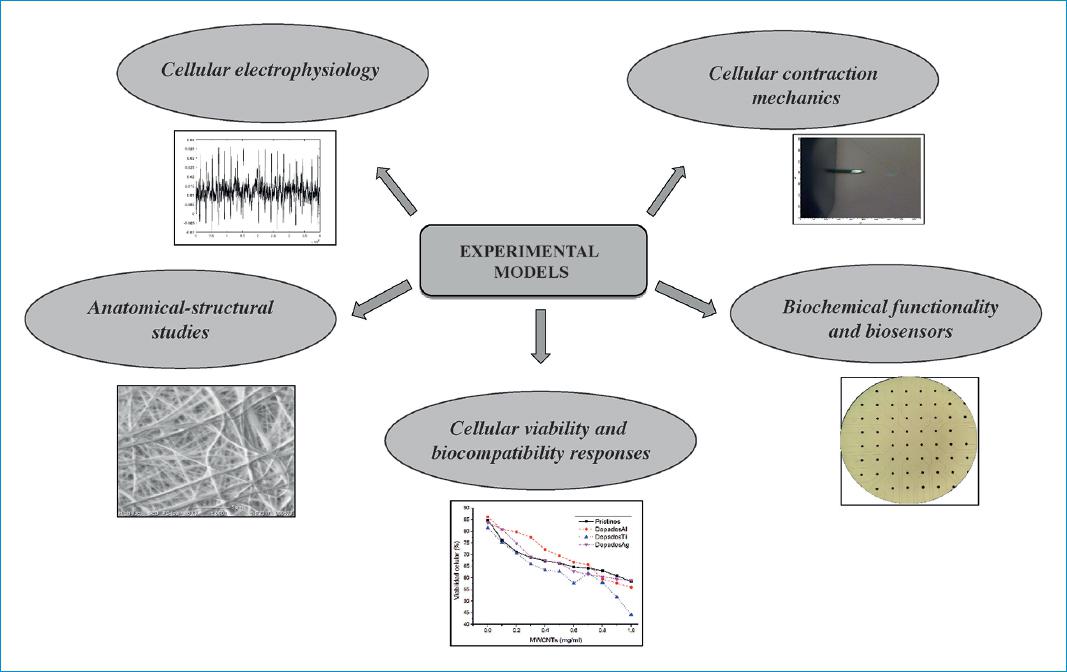
Given the versatility offered by in vitro models to carry out different kinds of studies, there are multiple lines of work which have emerged in relation to these research approaches in the field of cardiology. They include research on various bioelectric, biomechanical and biochemical phenomena, as well as applied research focused on the determination of responses to various drugs and the use of different biomaterials24 (Fig. 4).
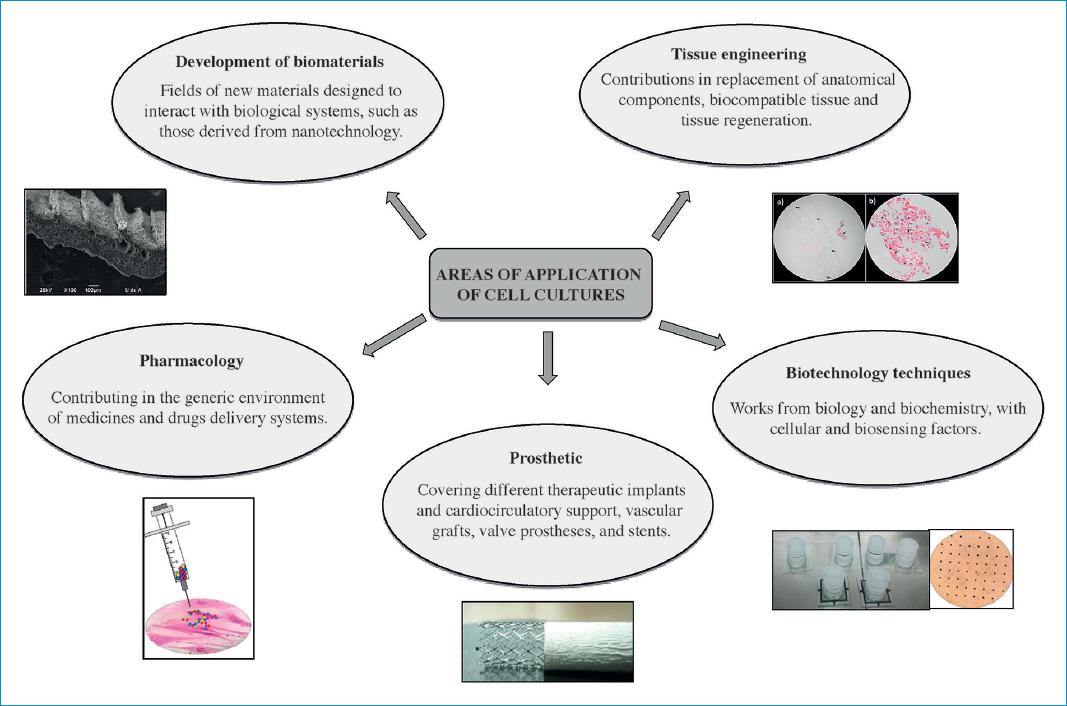
Figure 4 Areas of application for cardiovascular cell cultures.Source: Cardiovascular Dynamics Group, Universidad Pontificia Bolivariana.
In this context, researchers regard cell culture studies as a source of relevant information which helps them understand biological phenomena in the cardiovascular area, whose explanation is determined by the structural and functional relationship of cells. These studies further offer the possibility to carry out interactions between cellular and intercellular structures, considering that these interactions are responsible for multiple tissue or organ functions, which makes them more complex than the individual study of each cell. Therefore, an interesting aspect that can be addressed with this methodology is to respond through dynamic observations during culture modifications, adjusting to adaptation mechanisms as a result of various circumstances and stimuli imposed by the study model25.
Studies on functional cellular interrelation
In the functional context of cardiac tissue, there are contractile and non-contractile cells. The latter include support and interconnection cells, which in turn include the so-called fibroblasts, the constituents of most part of the cardiac matrix architecture. Their function is to provide structural stability to the heart during its pumping activity, during which stresses are produced as a result of motion and recovery26. At the same time, in the face of myocardial lesions that trigger a fibrotic and pro-inflammatory response, phenotypic and biochemical activation of cardiac fibroblasts is generated. This is related to the increase in the synthesis of extracellular matrix proteins and growth factors in function to generate collagen fibrotic tissue27. Some research has demonstrated that the functionality of fibroblasts depends on local mechanical signals and anatomical location, which is related to the proliferative and fibrogenic capacity in cardiac tissue injury28.
Cardiomyocytes are excitable cells. Therefore, they respond to the electric conduction spontaneously originated in the autonomic centers and transfer it from cell to cell through gap junctions, which interconnect cell membranes. This enables the propagation of action potentials, causing activation through transmembrane ionic currents in each cell29. The propagation of such potential through the conduction paths, and then between each cell, is synchronized and generates the orderly activation of the different regions, in which represents the electromechanical coupling response that follows the periodic phenomenon of depolarization and repolarization of cardiac cells30.
In this functional framework, the cells specialized in mechanical work, or cardiomyocytes, exhibit a cyclical contraction due to the shortening of myosin filaments over actin filaments. This activity occurs in an environment of high energy consumption, which engages a series of metabolic and endocrine paths in which a large number of precursors are important to maintain cell functional integrity.
Pathologies and physiological events
Multiple cardiovascular dysfunctions can be studied using cell culture platforms: atrial or ventricular arrhythmias, conduction tissue blockage and contractile alterations, which may be mediated by metabolic alterations, tissue perfusion, inflammatory lesions, fibrosis, and other conditions, which may include myocardiopathies, congenital and acquired structural alterations, and coronary disease31.
New study fields emerge in the intervention with pharmacological therapies or the use of new materials, evaluating functional response and incidence of cell viability32. All this in the function of proposing alternative therapies that allow the bioelectrical and biomechanical functionality of the heart tissue, as well as allowing tissue regeneration processes through the use of fibrillar, spongy, and thermosensitive biomaterials. Which must emulate the native microarchitecture of cardiac tissue, favoring cell-binding sites from integrins, electromechanical coupling to allow continuous contractions, and tissue remodeling for the proliferation, alignment, and replacement of native cells33.
Experimental models
Electrophysiological models
Various cardiovascular bioelectrical phenomena take place at cell level. These include cardiac cell transmembrane potentials as well as action potentials traveling through the tissue. Different electrode microarray platforms are capable of capturing the weak signals generated, which can be amplified and filtered for a better analysis and interpretation. On these platforms, microelectrodes are placed in multiple-channel matrices which show the local stimuli in a two-dimensional plane, thus allowing the creation of maps showing the polarization and depolarization of the tissue under study34. These maps carry the signal amplitude data, the displacement velocity of the depolarization wave, and repolarization times. In relation to the three-dimensional study of electrical phenomena, it is possible to use mapping techniques with markers or tracers which track the changes following the polarization and depolarization of the cell membranes, so as to show the local stimuli in the three planes. Therefore, more complex maps can be created35 (Fig. 5).
Biomechanical models
The contractile activity of cardiac fibers establishes the tissue function known as pumping activity. This creates the need to study contractile forces and stress-strain relationships. Different test prototypes are used to study cell mechanical behavior, including atomic force microscopy (AFM) and micromechanical mechanisms such as the cantilever36. With this technique, the forces acting on a cell are sensed by means of the reaction observed after applying forces using the cantilever37. This technique allows the recording of the extracellular action potential signal and the synchronization of the contractile cycle with morphological and mechanical measurements. Aspects related to the hardening of cell membranes during the contraction process and softening during the relaxation phase38.
Biochemical models
Responses associated to secretion functions or others which generate different cell chemical reactions can be evaluated using biosensors and bioelectrodes that record biosignals, such as extracellular electrical potentials, which correspond to the sum of cell action potentials or different chemical activations in the metabolic environment, which are the result of functional activation39. Among the methods used are the determination of serum levels of sodium, potassium, calcium, magnesium, creatine phosphokinase, creatine kinase-MB, lactate dehydrogenase, highly sensitive C-reactive protein, and troponins. All these interactions determining the functionality of the cardiomyocytes and the severity of the lesion in the myocardial tissue40.
Viability and biocompatibility response models
Multiple cell responses, including cytotoxic, mutagenic, genotoxic, and viability responses, can be measured using different techniques. Viability can be determined through tests which evaluate cell vital functions. These tests include the Trypan blue exclusion technique or the Propidium iodide staining test41,42. To determine cytotoxicity, there are various tests to study the alterations in cell basic functions through colorimetry analyses, such as the 3(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide assay or the Kenacid blue binding method43. In addition, the Ames test or the chromosomal aberration test, which analyze mutagenic responses, is used to study mutations or genetic damage in cell cultures caused by external agents. Finally, the Comet assay or the micronucleus test are used to assess genotoxic damage44.
Anatomical-structural study models
The characterization and differentiation of cardiac tissue structures and substructures can be performed using different microscopy techniques, which show the functional architecture in a culture cell environment. The AFM is used to determine micro and nanostructures through a 3D surface topography scanning and the study of the mechanical properties of cells45. The Raman spectroscopy uses the vibration of molecules in the structure to analyze the structural modifications of biomaterials interacting with cell cultures46. Finally, the scanning and transmission electron microscopies, respectively, are used to study the ultrastructure of cell cultures, from its superficial components to its internal composition47.
About the current situation at a regional level
The implementation of different techniques and the development of technological devices in basic research, coupled with the emergence of new research fields, have driven the creation of new groups who are expert in handing cell cultures for various purposes. However, considering the current situation in our region, the critical mass of resources has not significantly increased. Unfortunately, this is due to the high costs required to maintain cutting-edge technology in this area, which includes the enabling technical areas of work and the assembly of a series of tools which render its implementation difficult, in addition to a lack of training and qualifications of research staff capable of interweaving elements ranging from strictly biological concepts to strictly clinical ones.
In our region, the fact that only few expert groups are exclusively devoted to the study of cell platforms in the field of cardiology has reduced the possibilities offered by the method. Consequently, inter-institutional and transnational collaborations and networks should be promoted to motivate the research community and provide scientific and technological support to carry out high-level projects on physiological and pathophysiological cell components, focused on the cardiovascular field. This strategic relationship will enable collaborative work, thus creating a scientific support scheme and expanding the research base to implement high-level platforms which offer technological advantages, while considering costs, opportunity, and quality elements.
Conclusions
In the cell culture field, the cardiovascular medicine found a method to understand the biological phenomena taking place in the heart and the blood vessels, which are determined by the structural and functional relationship between cells and tissue. This method can then be used to study the responses in the environment through observations during modifications, by means of adjustments to adaptation mechanisms caused by various stimuli imposed by the study model. Therefore, cell cultures provide a new development and application field which will extend and offer new highly relevant research possibilities in the cardiovascular area. As long as disciplines such as biology, chemistry, biomedical engineering or bioengineering and clinical sciences work collaboratively with each other, the field of cardiovascular medicine may become an area of great challenges and achievements aimed to benefit patients.