Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230On-line version ISSN 2422-2844
Rev.fac.ing.univ. Antioquia no.52 Medellín Apr./June 2010
Concrete deterioration in Colombian urban atmospheres
Deterioro del concreto en ambientes urbanos de Colombia
Esteban Correa*, Sergio Peñaranda, Juan Castaño, Félix Echeverría
Grupo de Corrosión y Protección, Universidad de Antioquia - Sede de Investigación Universitaria (SIU). Calle 62 No 52-59. Torre 2 Lab. 330, Medellín, Colombia
Abstract
The combination of atmospheric pollutants, such as carbon dioxide and chlorides has a synergistic effect accelerating the degradation process of concrete. However, in Colombia it has not been carried out field tests in real urban environments to assess the deterioration of concrete and its relation to both, carbonation and chloride content. In this work, cylindrical concrete probes were exposed in different urban atmospheres with the aim of establishing correlations between the concrete deterioration and the type of atmosphere. To evaluate the corrosion rate on the rebar and the percentage of carbonated probe, Electrochemical Impedance Spectroscopy (EIS) and physicochemical tests with phenolphthalein were used, respectively.
Keywords: Concrete, atmospheric deterioration, carbon dioxide, chlorides, EIS
Resumen
La combinación de contaminantes atmosféricos tales como el dióxido de carbono y los iones cloruro tiene un efecto sinérgico que acelera los procesos de degradación del concreto. Sin embargo, en Colombia no se han llevado a cabo estudios de campo en ambiente urbanos reales que permitan evaluar el deterioro del concreto y su relación con la carbonatación y el contenido de iones cloruro. En este trabajo, probetas cilíndricas de concreto fueron expuestas en diferentes atmósferas urbanas con el ánimo de establecer correlaciones entre el deterioro y el tipo de atmósferas. Para evaluar la velocidad de corrosión de la barra de refuerzo y el porcentaje de carbonatación de la probeta se empleó la Espectroscopía de Impedancia Electroquímica (EIS) y ensayos fisicoquímicos con fenolftaleína, respectivamente.
Palabras clave: Concreto, deterioro atmosférico, dióxido de carbono, cloruros, EIS
Introduction
Rebar protection provide by the concrete is improved by high pH values reached after the hydration reactions. In this alkaline environment, the steel is normally protected from corrosion by the formation of a passive oxide film which acts as a barrier to the anodic dissolution of the metal [1, 2]. However, the passivity breakdown occurred when the structure interacts with the atmosphere [3]. Steel rebar can be contaminated by chloride ions from sea spray or windblown salt. This fact leads to the formation of corrosion products on their surface [4]. The presence of the corrosion products affects the bond strength between steel and concrete [4, 5]. Another aggressive agent for the concrete structures is the atmospheric carbon dioxide which reacts with the humidity and produces species with lower pH values such as calcium hydroxide. This phenomenon is known as carbonation. The carbonation of concrete reduces the initially high pH value of the pore solution phase to a level at which the passive film can breakdown, allowing the steel to corrode [5-7]. The combination of these agents has a synergistic effect accelerating the degradation process [8]. No studies have been carried out in Colombia in real urban environments to assess the concrete deterioration and its relationship to carbonation and chloride content. The research described in this paper includes electrochemical and physicochemical tests to evaluate the impact of aggressive agents, such as atmospheric carbon dioxide and chloride ions on mortar specimens. The aim of the present work is to look for correlations between the concrete deterioration and the type of atmospheres after exposures in different Colombian urban sites. For this end, cylindrical reinforced and non-reinforced probes were built and exposed to different urban atmospheres. To evaluate the corrosion rate on the rebar and the percentage of carbonated probe, Electrochemical Impedance Spectroscopy (EIS) and physicochemical test with phenolphthalein were used, respectively.
Methodology
Field tests were performed in three cities with different characteristics (altitude over sea level, distance to the coast and population). Table 1 shows the main characteristics of these cities. Three monitoring stations were placed in each city for the concrete exposures. The cites were classified in terms of its economical activity (industrial, commercial or residential). A total of nine urban monitoring stations were used.
Table 1 Characteristics of the cities selected for field test
On each station, 7 probes were installed as follows: 4 reinforced and 3 non-reinforced, for a total of 63 mortar probes (27 non-reinforced and 36 reinforced) with dimension of 5 inches on length and 2 inches on diameter. Type I cement Portland, standard sand with granulometry according to ASTM C778 [9], water and structural steel according to ASTM 706 [10] with 1.5 cm of diameter were used to build the probes.
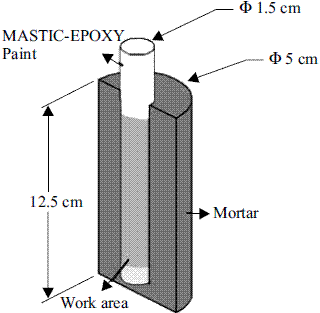
For the reinforced probes, the rebar was cut into 14 cm sections. Besides, both ends of the rebar and the mortar-rebar-air interface were covered with a mastic-epoxy system to reduce or eliminate the anodic spots, leaving a free surface of about 40 cm2. Water/cement ratio of 0.6 and sand/cement ratio of 3 were used to fabricate the mortar specimens. Figure 1 shows the configuration and dimensions of the mortar probes. Thereafter, the rebars were positioned vertically at the center of the moulds, where the mortar were cast and stored at ambient conditions in the laboratory for 24 h. After being dismolded, the specimens were placed in a curing room (RH > 80%, T = 22 ± 1 oC) for a month. At the end of the curing period, the probes were evaluated on the different urban environments every 4 months, during one year.
Figure 1 Diagram of mortar probes
A BAS-ZAHNER IM6e potentiostat/galvanostat, saturated calomel electrode (SCE) as reference electrode, deionized water as electrolyte and platinum wire as counter electrode were used to take EIS measurements. Before each measurement, the probes were kept inside the electrolyte during 30 minutes to stabilize the potential and then scanning frequency measurements between 10 mHZ to 100 KHz were performed. For open circuit potential measurements, a SCE having a wetted cotton tip was placed over the concrete surface and the potential was periodically measured between the rebar and the reference electrode using a FLUKE high impedance multimeter. Finally, to determine the area of carbonation, 3 cm sections were cut and a phenolphthalein solution was poured on the surface.
Results and discussion
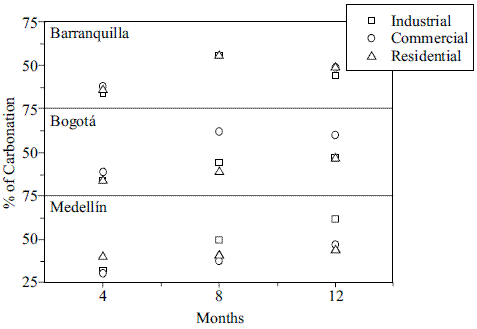
Figure 2 shows the average area of carbonation (in percentage) for the specimens on each urban station after 4, 8, and 12 months. Measurements of the carbonation profile varied as follows: 31 - 40 % for the first 4 months, 38 - 62 % after 8 months and 44 - 62 % after 12 months.
Figure 2 Percentage of carbonation on each urban station after 4, 8, and 12 months exposure
In Barranquilla, the progress of the carbonation was similar for each station. It was higher between 0 and 4 months and then levels off. According with these results, Barranquilla station showed a similar aggressiveness behavior for the mortar probes.
The results in all Bogotá stations showed an increasing carbonation progress. The behavior was similar in both, residential and industrial station. Commercial site showed greater carbonation because this area has a very high CO2 concentration probably due to a heavy vehicular traffic.
In the case of Medellín, the behavior was different for each station. In the last two periods, the progress of carbonation in industrial stations was clearly higher than in the other two stations.
Carbonation phenomenon could changes the properties of concrete, specifically the mechanical and electrical resistance. As the carbonation progresses, hydroxides in solution (calcium, sodium and potassium) present in the pores of the mortar are converted into solid nonsoluble carbonates. These carbonates fill the pores, decreasing the mortar porosity. The lower porosity causes a rise of mortar density, which improves some properties such as compressive strength, hardness and elastic yield. However, the rise of the mortar density may have a negative effect because of the ductility and the adhesion between rebar and mortar decreases. On the other hand, carbonation reduces the charge transport capacity by reducing the concentration of ions available for that purpose. In the same way, lower mortar porosity produces an increase on the mortar resistivity; because there is a reduction in sites where charge can be transported [6, 11, 12].
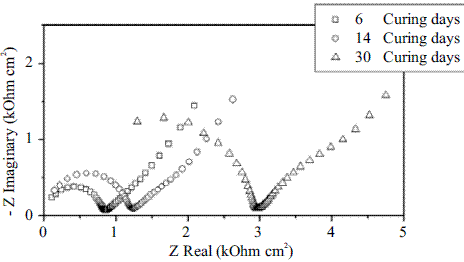
EIS tests were carried out to determine the rebar behavior in mortar. Figure 3 shows the Nyquist diagram obtained during the curing period for a reinforced probe. A similar geometry was observed in each plot, a capacitive arc at high frequencies and linear behavior at low frequencies. In addition, there was an increase in the capacitive arc with time of curing. In this case, the arc at high frequencies, describes the behavior of mortar (pore solution resistance and mortar resistance) and its increase with curing time. These may be attributed to microstructural change (mortar hardening and a rise in mechanical strength) due to mortar hydration, according with Koleva et al [13]. On the other way, at low frequencies, Nyquist plot shows a linear behavior. This behavior results from the diffusion of oxygen through the mortar towards the steel/mortar interface because the diffusion process is the controlling process in this situation [14].
Figure 3 Nyquist diagrams obtained during curing period for a reinforced probe
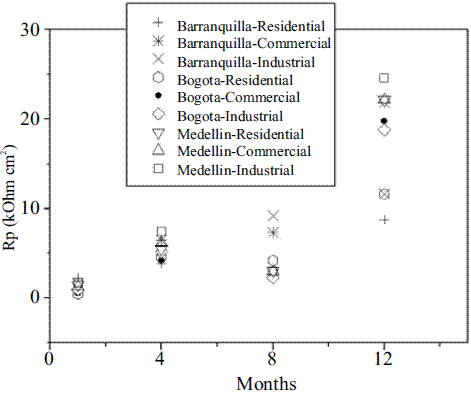
Figure 4 shows the concrete pore resistance values (capacitive arc diameter at high frequencies) taken from Nyquist diagrams and its trend with exposure time. It is noted that the pore resistance values measured tend to increase with increasing exposure time. Diminution of the mortar density and loss of the charge transport capacity, increase the mortar resistance (pore solution resistance and mortar resistance) due to carbonation progress, as explained above.
Figure 4 Reinforced concrete pore resistance vs. Exposure time for each urban area
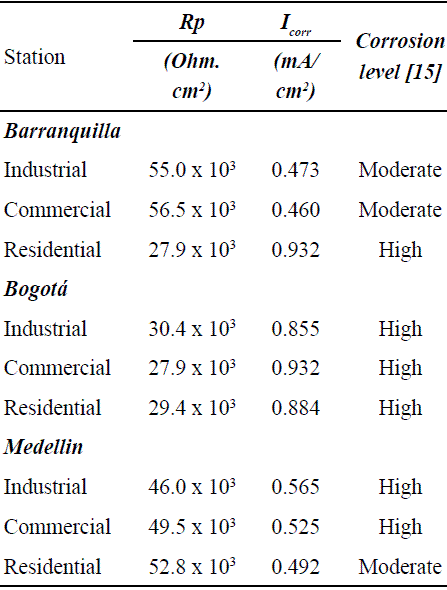
Table 2 shows the polarization resistance values (Rp) taken from the last period and the corrosion rate (Icorr) determined by the Stern-Gary [15] equation for each station.
Table 2 Polarization resistance and corrosion rate for the last period on each urban place
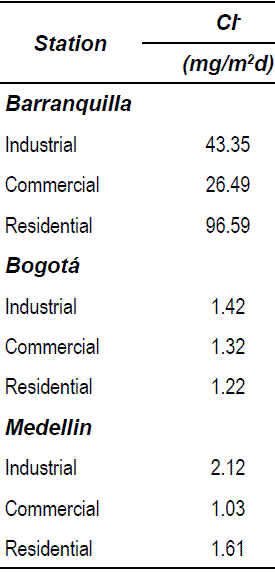
Table 3 presents the average chloride concentration measured in the 9 stations during the same year [16]. In the residential station of Barranquilla, placed near to the seashore, a high chloride concentration was measured, which means a high aggressiveness environment. In this case, both the carbonation progress as well as the high chloride concentration may cause breakage of the steel passive layer, which could explain the elevate corrosion rate obtained through EIS. According to Table 3, in Bogota city, all the sites showed a similar corrosion level, exhibiting a corrosion rate slightly higher at the commercial station. This is in agreement with the results obtained in carbonation test, where the station had the highest percentage of carbonation progress. In this case, the rebar deterioration was due to mortar carbonation because the concentration of chloride was below 2 mg/m2.day, corresponding to atmospheres with low chlorides concentration, according to ISO classification [17].
Table 3 Average chloride concentration for one year of exposure
The results obtained for Medellín city show high corrosion levels for commercial and industrial stations. For residential area, the level is moderate. Deterioration of rebar in Medellín stations was due mostly to carbonation process because the concentration of chloride was very low.
Acknowledgments
Authors express their gratitude to COLCIENCIAS and CREG -Comisión Reguladora de Energía y Gas- for the financial support to the project 115- 06-17398.
Conclusions
The highest corrosion rate were presented in residential station of Barranquilla, which contained high concentration of chloride ions in the air, and in all Bogotá stations, where the main source of deterioration of the rebar was the carbonation of mortar.
The impedance curves showed a capacitive loop associated to mortar behavior (resistance of the pore solution and resistance of mortar) during curing. The increase of the impedance with time could be attributed to micro-structural changes due to mortar hydration. Also, low frequencies tails were noted, which were associated with double layer phenomena on the rebar surface.
References
1. D. J. Anstice, C. L. Page, M. M. Page. The pore solution phase of carbonated cement pastes. Cement and Concrete Research. 2005. Vol. 35. pp. 377-383. [ Links ]
2. P. Venkatesan, N. Palaniswamya, K. Rajagopal. Corrosion performance of coated reinforcing bars embedded in concrete and exposed to natural marine environment. Progress in Organic Coatings. 2006. 56. pp. 8-12. [ Links ]
3. M. Moreno, W. Morris, M. G. Alvarez, G. S. Duffó. Corrosion of reinforcing steel in simulated concrete pore solutions Effect of carbonation and chloride content. Corrosion Science. 2004. 46. pp. 2681-2699. [ Links ]
4. B. Díaz, X. R. Nóvoa, M. C. Pérez. Study of the chloride diffusion in mortar: A new method of determining diffusion coefficients based on impedance measurements. Cement and Concrete Composites. 2006. Vol. 28. pp. 237-245. [ Links ]
5. G. Batis, E. Rakanta. Corrosion of steel reinforcement due to atmospheric pollution. Cement and Concrete Composites. 2005. Vol. 27. pp. 269-275. [ Links ]
6. J. Xiao, J. Li, B. Zhu, Z. Fan. Experimental study on strength and ductility of carbonated concrete elements. Construction and Building Materials. 2002. Vol. 16. pp. 187-192. [ Links ]
7. M. Sánchez, J. Gregori, C. Alonso, J.J. García-Jareño, H. Takenouti, F. Vicente. Electrochemical impedance spectroscopy for studying passive layers on steel rebars immersed in alkaline solutions simulating concrete pores. Electrochimica Acta. 2007. Vol. 52. pp. 7634-7641. [ Links ]
8. H. Song, S. Kwon, K. Byun, C. Park. Predicting carbonation in early-aged cracked concrete. Cement and Concrete Research. 2006. Vol. 36. pp. 979-989. [ Links ]
9. ASTM. Standard Specification for Standard Sand. West Conshohocken. PA. ASTM (Standard: ASTM C778). 2006. pp. 1-3. [ Links ]
10. ASTM. Standard Specification for Low-Alloy Steel Deformed and Plain Bars for Concrete Reinforcement. West Conshohocken. PA. ASTM (Standard: ASTM 706/A 706/M). 2008. pp. 1-5. [ Links ]
11. R. Polder, C. Andrade, B. Elsener, O. Vennesland, J. Gulikers, R. Weidert, M. Raupach. Test methods for on site measurement of resistivity of concrete. Materials and Structures, 2000. Vol. 33. pp. 603-611. [ Links ]
12. J. Chang, W. Yeih, R. Huang, J. Chi. Mechanical Properties of Carbonated Concrete. Journal of the Chinese Institute of Engineers. 2003. Vol. 26. pp. 513- 522. [ Links ]
13. D. A. Koleva, J. Hu, A. L. A. Fraaij, P. Stroeven, N. Boshkov, J. H. W. de Wit. Quantitative characterization of steel/cement paste interface microstructure and corrosion phenomena in mortars suffering from chloride attack. Corrosion Science. 2006. Vol. 48. pp. 4001-4019. [ Links ]
14. G. Qiao, J, Ou. Corrosion monitoring of reinforcing steel in cement mortar by EIS and ENA. Electrochimica Acta. 2007. Vol. 52. pp. 8008-8019. [ Links ]
15. O. Trocónis. Manual de inspección, evaluación y diagnostico de corrosión en estructuras de hormigón armado.Cyted-Red Durar. 1997. pp. 134. [ Links ]
16. Grupo de Corrosión y Protección. Impacto de la corrosividad atmosférica sobre la infraestructura del SEC y sobre los cotos AOM. Informe Final. Código Proyecto 115-06-17398. 2008. Universidad de Antioquia. Medellín. pp. 113. [ Links ]
17. ISO. Corrosion of metals and alloys - Corrosivity of atmospheres - Classsification. Geneva. Switzerland. ISO (Standard: ISO 9223). 1992. pp. 1-15. [ Links ]
(Recibido el 20 de mayo de 2009. Aceptado el 23 de septiembre de 2009)
*Autor de correspondencia: teléfono: + 57 + 4 + 219 66 17, fax: + 57 + 4 + 219 65 65, correo electrónico: estebancorrea@udea.edu.co (E. Correa)