Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230On-line version ISSN 2422-2844
Rev.fac.ing.univ. Antioquia no.57 Medellín Jan./March 2011
Preparation and characterization of Mg-modified zirconias as catalysts for the direct synthesis of dimethyl carbonate (DMC)
Preparación y caracterización de circonias modificadas con Mg como catalizadores para la síntesis directa de carbonato de dimetilo (DMC)
Andrés Orrego Romero, Consuelo Montes de Correa* , Felipe Bustamante Lodoño
Environmental Catalysis Research Group. Sede de Investigación Universitaria. Universidad de Antioquia. Calle 53 N.° 61-30. Medellín. Colombia
Abstract
In this work sol-gel zirconia was prepared using H2SO4, HNO3 or HCl as hydrolysis catalysts. Basic and acid sites of synthesized zirconia materials were characterized by XRD, BET, FTIR, CO2- TPD and NH3-TPD. FTIR spectra and TGA confirmed the presence of sulfate in the structure of samples prepared with H2SO4 as hydrolysis catalyst. Different molar ratios of Mg were incorporated by co-gellation on selected zirconia materials in order to improve their basic properties. Mg loading was determined by elemental analysis. The resulting materials were tested for the direct synthesis of dimethyl carbonate (DMC) from methanol and CO2. The addition of Mg to zirconia samples prepared with HCl as hydrolysis catalyst increased their CO2 adsorption capacity between 100 and 200°C. However, methanol conversions on these samples were lower than over unmodified zirconia samples.
Keywords: Sol-gel process, zirconia, basic sites, dimethyl carbonate, CO2-TPD.
Resumen
Se preparó circonia por el método sol-gel usando H2SO4, HNO3 o HCl como catalizadores de hidrólisis. Los materiales resultantes se caracterizaron por DRX, BET, FTIR, desorción de CO2 con temperatura programada (TPD- CO2) y desorción de amoníaco con temperatura programada (TPD-NH3). Los espectros FTIR de las muestras preparadas con H2SO4 muestran bandas típicas de sulfatos, lo cual se confirmó mediante TGA. Adicionalmente, en algunos materiales seleccionados se incorporaron diferentes proporciones de Mg mediante co-gelación. La carga de magnesio de los materiales modificados se determinó por análisis elemental. Los materiales se ensayaron como catalizadores en la síntesis directa de carbonato de dimetilo (DMC) a partir de metanol y CO2. La incorporación de Mg incrementó la capacidad de adsorción de CO2 entre 100 y 200°C. No obstante, las conversiones de metanol obtenidas con estas muestras fueron menores a las de las muestras de circonia sin modificar.
Palabras clave: Método sol-gel, circonia, sitios básicos, carbonato de dimetilo, TPD-CO2, TPD-NH3.
Introduction
The interest in the production of dimethyl carbonate (DMC), a compound catalogued as a "green chemical", has been growing over the last decade. DMC is an environmentally friendly raw material of wide versatility and a possible substitute of highly corrosive or toxic reagents in organic synthesis such as: dimethyl sulfate, chloromethane and phosgene. Moreover, DMC can act as methylating and carbonylating agent or as an intermediate in the production of higher carbonates, polyurethanes, isocyanates, polycarbonates and other fine chemicals [1]. Due to its high oxygen content and reduced environmental impact, DMC has also emerged as a potential substitute for methyl tertiary butyl ether (MTBE), used as an oxygenated fuel additive (i.e., octane enhancer) [2]. The direct synthesis of DMC from methanol and CO2 (reaction 1), which would replace the traditional synthesis via the phosgenation of methanol, is an attractive route for its production [3].
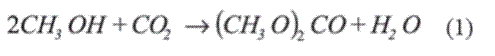
Several catalysts have been reported for the one-step synthesis of DMC (reaction 1) [4]: organometallic compounds, Ni and Ti tetraalkoxides, K2CO3, ZrO2, H3PW12O4-ZrO2, H3PO4- V2O5, Cu-Ni/VSO, Cu-Ni/CA [5], Rh/ZSM-5 [6]. However, DMC yields are still very low, even in the presence of dehydrating agents (to shift the reaction towards products) and additives. Furthermore, almost all catalytic experiments have been carried out in the liquid phase under high pressure conditions (supercritical conditions) showing disadvantages versus gasphase processes in terms of controlling the process variables, catalyst recovery and associated costs.
Previous studies [7, 8] have shown the simultaneous presence of acid and basic sites on zirconia, which are believed to be the active sites for the direct synthesis of DMC. However, low methanol conversions have been obtained (less than 1%) due to the difficulty of CO2 activation. Those reports also emphasized that the strength of the basic sites would favor CO2 activation in that reaction [9]. The use of metal oxides as dopants is proposed not only to improve the chemical stability but also, to increase the oxygen anion concentration and basicity of zirconia [10].
The synthesis of sol-gel zirconia materials has been previously reported. Montes et. al. [11] reported a 100% tetragonal phase using H2SO4 while about 63.6% tetragonal phase was obtained using HNO3. At a calcination temperature of 600 °C crystalline materials with defined properties and surface areas up to 67 m2/g were obtained. On the other hand, Bokhimi et al. [12] mostly obtained the monoclinic phase (97%) when HCl was used as hydrolysis catalyst and the material was calcined at 800 °C.
In this paper magnesium oxide -which displays CO2 adsorption capacity [13] - was added by cogellation to several sol-gel zirconia materials. It was found that Mg increases the CO2 adsorption capacity of zirconia in the temperature range (100 - 200°C) where the formation of DMC occurs. However, methanol conversions to DMC over Mg modified zirconia are lower than on unmodified zirconia materials.
Experimental
Catalyst preparation
Zirconia was prepared by modifications of previously reported methods [11, 12]. Solution A, containing 10 mL of zirconium (IV) butoxide (Aldrich) Zr(OC4H9)4 in 85 mL of isopropanol (J.T. Baker), was stirred at room temperature. Then HCl (37%, Merck), HNO3 (65%, Merck) or H2SO4 (95-98%, Merck) was added to solution A in order to favor alkoxide hydrolysis. After aging the gel for 24 h at room temperature without stirring, solution B, containing 5 mL of H2O and 42 mL of isopropanol, was added drop-wise to solution A under vigorous stirring until gellation. Finally, the gel was dried at 100 °C during 60 h. The samples prepared using H2SO4, HNO3 and HCl were coded as ZrO2-S, ZrO2-N and ZrO2-C, respectively.
Magnesium-modified zirconia was prepared as follows: about 5.5 g of Mg (NO3)2 • 6H2O (Merck) were dissolved in solution B, prior to mixing with solution A. The Mg/Zr molar ratio was 1. The resulting magnesium-loaded materials were coded: Mg/ZrO2-N (prepared with HNO3) and Mg/ZrO2-S (prepared with H2SO4). Both materials were calcined in a static air furnace at 600 °C (873 K) and 800 °C (1073 K) for two hours at a heating rate of 1 °C/min. The highest calcination temperature was used only for comparison purposes. Other materials were prepared by varying Mg loading following a similar procedure, but using HCl as initiator of the alkoxide acid hydrolysis. These samples were calcined at 800 °C in order to obtain a stabilized monoclinic phase as reported by Bokhimi [12]: The Mg/Zr molar ratios were 0, 0.1, 0.5 and 1. The codes of the samples are summarized in table 1.
Characterization
The crystallinity of synthesized materials was determined by X-ray diffraction (XRD) on a Phillips PW 1710 diffractometer using Cu Kα radiation and Ni filter operated at 30 kV and 20 mA at room temperature. The scanning range was 2θ= 20-60°, step of 0.014°. Magnesium loading was determined by atomic absorption spectroscopy (AAS) on a Philips PU9200. Specific surface areas were determined by N2 adsorption at 77 K in an AutoChem II 2920 (Micromeritics).
The basic and acid sites of the samples were characterized by temperature programmed desorption using 99.9% CO2/He and 0.3% NH3/He, respectively. TPDs were performed in a Micromeritics AutoChem II 2920 apparatus coupled to a mass spectrometer (Thermostar- QMS 200, Pfeiffer Vacuum), where the mass signals m/e- 44 (basic sites) and m/e- 17 (acid sites) were monitored. In addition, signals m/e- 30 for the decomposition of anions NO3- and m/e- 64 for the decomposition of sulphate anions SO4-2 were monitored. Samples (0.1 g) were pretreated in 25 mL/min flowing He (99.99%) up to 500 °C for 1 hour at 10 ° C/min. Samples were then cooled to 50 °C and saturated with 50 mL/min CO2 (basic sites) or NH3 (acid sites) for 1 hour. CO2 or NH3 loosely bound to the surface were removed by flowing 50 mL/min of He for 1 hour at 10°C/min up to 900 °C. FTIR spectra of selected samples pressed in KBr pellets were collected with a Nicolet Avatar 330 spectrometer. The sulfur content of samples prepared with H2SO4 was determined by TGA using a 2950 TGA/HRV6 instrument where the sample was heated up to 950 °C at 10 °C/min in flowing nitrogen (100 mL/min).
Catalytic tests
Catalytic tests were performed in a tubular quartz reactor (ID 10 mm) packed with 0.5 g of catalyst sample. The products were analyzed using a mass spectrometer QMS Thermostar 200 (Pfeiffer) with a resolution of 0.01 ppm. The reaction mixture consisted of a 2:1 molar ratio of CO2 (99.9%) and methanol-saturated helium (grade UAP). The reactions were carried out at 80, 120, 160, and 200 °C for 60 min. The reactor pressure was 720 mmHg, and the total flow was maintained about 20 mL/min.
Results and discussion
Catalyst characterization
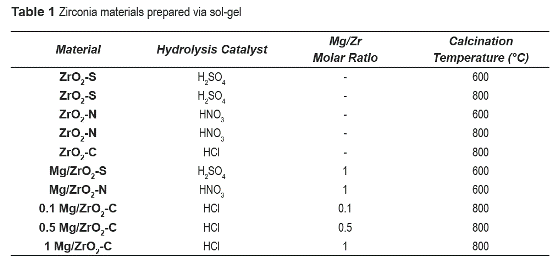
XRD patterns of figure 1 indicate that ZrO2-N and ZrO2-S are crystalline. Sulfated zirconia, ZrO2-S exhibits a tetragonal phase (diffraction peaks at 2θ = 30.5, 35.5, 50°), whereas two phases, monoclinic (diffraction peaks 2θ = 28.5 and 31.5°) and tetragonal were identified in ZrO2-N [14]. Magnesium-modified zirconia materials, Mg/ZrO2-S and Mg/ZrO2-N, were amorphous and no diffraction peaks were observed in the 20 interval examined. This change of crystallinity has been reported by Liu et. al. [15], where MgO was incorporated on sol-gel zirconia samples. For different MgO loadings only appreciable diffraction peaks were observed at 2θ = 1 0. Those materials, which are described as mesoporous, were strongly influenced by MgO incorporation. Their observations were explained in terms of the solubility of the magnesium precursor salt during the sol-gel process, specifically on hydrolysis/ condensation of zirconium alkoxide and the self- assembly of the gel. Thus, excessive magnesium salt added to the system could influence or even damage the formation of the mesoporous framework. As can be observed in figure 1, Mg/ZrO2-S and Mg/ZrO2-N show no XRD peaks indicating that the tetragonal structure disappeared.
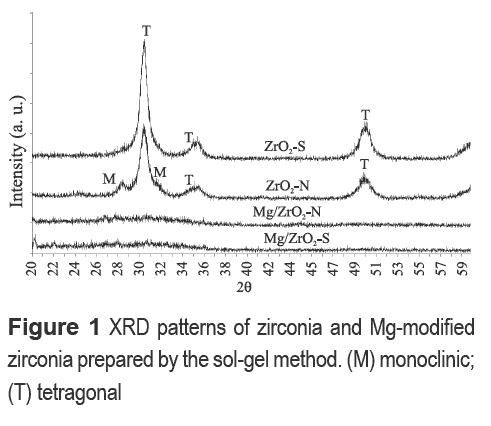
As shown in figure 2, ZrO2-C synthesized using HCl as hydrolysis catalyst and calcined at 800 °C showed diffraction peaks at 2θ = 28.5 and 31.5° associated with the monoclinic phase. However, incorporation of Mg to zirconia samples prepared with HCl as hydrolysis catalyst resulted in crystalline materials with predominantly tetragonal phase. It can be noticed in figure 2 that increasing calcination temperature to 800 °C and using HCl as hydrolysis catalyst for the solgel synthesis of zirconia improves crystallinity of the Mg-containing materials. Jung and Bell [8] reported that either monoclinic or tetragonal zirconia, or a mixture of both, can catalyze the reaction of methanol and CO2 to DMC. In this sense, the purpose of this study was to determine whether Mg incorporation on zirconia would improve conversion of methanol to dimethyl carbonate (DMC). ZrO2-N samples were calcined at 800 °C to compare their crystallinity with ZrO2-C samples (also calcined at 800 °C). XRD pattern for ZrO2-N (calcined at 600 °C) shows the presence of both crystalline phases, monoclinic and tetragonal. The increase in the calcination temperature from 600 to 800 °C did not significantly modify the crystalline phase of ZrO2-N samples but, the surface area decreased (see table 2).
Surface area and Mg content
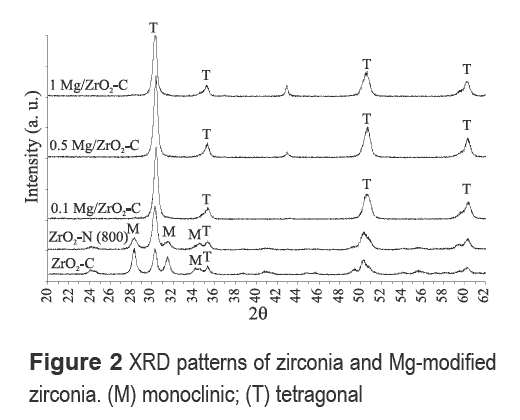
Table 2 lists the surface area of Mg-modified zirconia materials. ZrO2-N and ZrO2-S have higher surface areas than their Mg-modified counterparts. The surface areas of ZrO2-N and ZrO2-S samples decreased when calcination temperature increased to 800 °C. In general, materials prepared with different Mg loadings showed lower surface areas than zirconia samples synthesized with nitric or sulfuric acid.
CO2-TPD
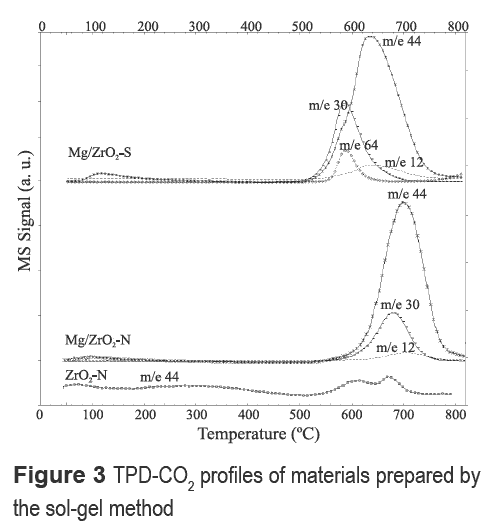
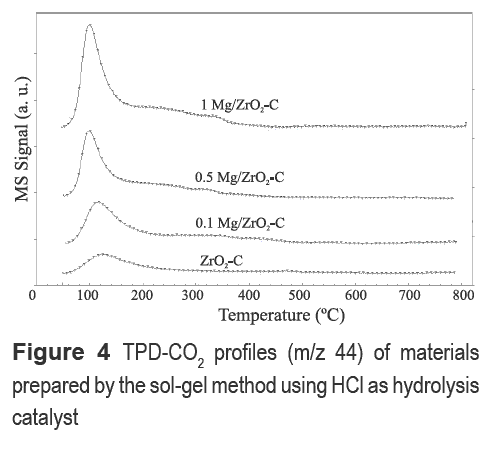
The intensity and the relative strength of basic sites were examined by CO2-TPD (figures 3 and 4). The CO2 desorption profiles of Mg-containing materials synthesized with HNO3 and H2SO4, respectively, are shown in figure 3. Peaks at 640 and 700 °C suggest the presence of strong basic sites. Furthermore, a significant enhancement of CO2 adsorption capacity is obtained on Mg- modified zirconia. ZrO2-N profile also revealed strong basic sites at 600 and 680 °C but CO2 adsorption capacity was lower; ZrO2-N shows a faint line of CO2 desorption between 100-400 °C, a somewhat similar behavior to Mg/ZrO2-N.
Mg/ZrO2-N shows a signal at 580 °C for m/e- 30 assigned to the decomposition of NO3- either from the Mg precursor or nitric acid. Mg/ZrO2-S showed weak basic sites around 100°C with very low adsorption capacity in addition to decomposition of nitrate and sulfate anions related to signals m/e-30 and m/e- 64, respectively, around 580 °C. From figures 3 and 4 it can be noticed that the use of HCl during the sol-gel synthesis of zirconia (ZrO2-C and Mg/ZrO2-C) affects the strength of the basic sites of the resulting material, since desorption peaks shift to lower temperatures. Moreover, CO2 adsorption in the region between 100 °C and 200 °C increases with Mg content.
NH3-TPD
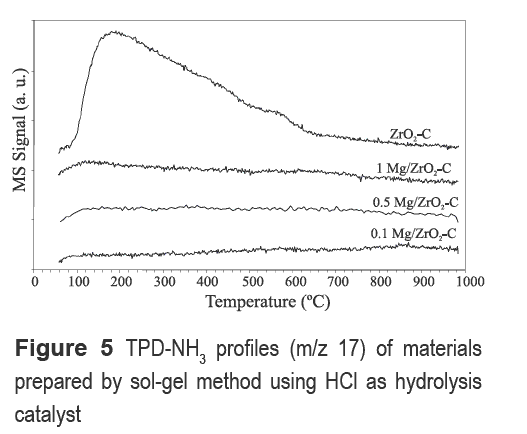
Figure 5 shows the NH3-TPD profiles of magnesium-modified zirconia samples using HCl as hydrolysis catalyst. The NH3-TPD profiles indicate that the acid sites of zirconia are affected by Mg incorporation. A broad region of NH3 desorption between 50 and 600°C is evidenced in the trace corresponding to unmodified zirconia -indicating the presence of acid sites- whereas none of the Mg-modified materials (different Mg loadings) show NH3 desorption signals in the temperature range examined. Since the surface area of ZrO2-C decreased by the addition of Mg (see table 2), it may be possible that surface Mg could block NH3 access to zirconia acid sites.
Thermal analysis
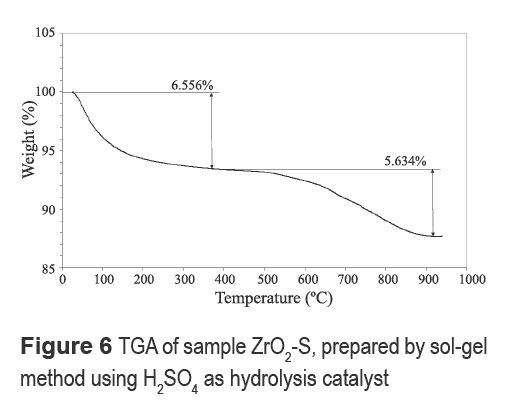
Figure 6 shows the TGA profile of a ZrO2-S sample. Two major weight loss regions are identified. The first weight loss of 6.5% observed up to about 400 °C is associated with the release of physisorbed water with a minor contribution from oxidation of organic species. The second weight loss of about 5.6% is observed above 500 °C and is attributed to decomposition of surface sulfate [17].
FTIR
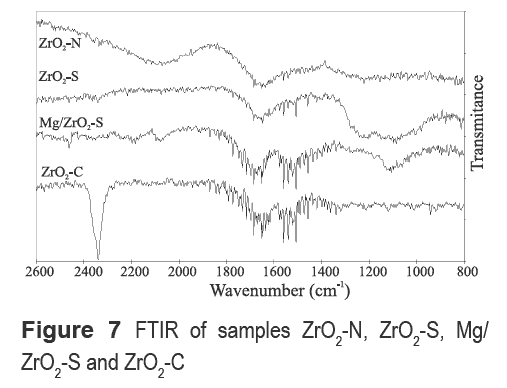
Figure 7 shows the FTIR spectra of samples calcined at 600 °C. ZrO2-S and Mg/ZrO2-S samples have a common broad band located at 1120 cm-1 assigned to the presence of surface sulfate complex in a bidentate configuration [17]. Furthermore, the spectrum of ZrO2-S shows a broad peak at 1223 cm-1 typical of a bidentate sulfate ion coordinated to a metal cation [16, 18]. ZrO2-N and ZrO2-C samples show no bands in the region from 800 cm-1 to 1300 cm-1. Spectra for sample ZrO2-C showed a very intense and sharp band at 2340 cm-1. This band has been assigned to CO2 trapped inside the bulk structure of the oxide [19]. Bands between 1550-1556 cm-1 have been assigned to the stretching vibrations of Zr-O and between 1637-1642 cm-1 (observed in all samples from Fig. 7) are attributed to stretching and flexion vibration of hydroxyl from water. [16].
Catalytic experiments
Only crystalline synthesized materials which exhibited basic sites in the range of 100-200°C (temperature region where high DMC yields have been obtained over zirconia) were tested as catalysts for the reaction between methanol and CO2. Thence, only ZrO2-C and Mg/ZrO2-C catalysts series were used. Zirconia samples synthesized with HNO3 or H2SO4 and modified with Mg were not tested because they showed no defined morphology, and high strength basic sites (CO2 desorbed above 600 °C).
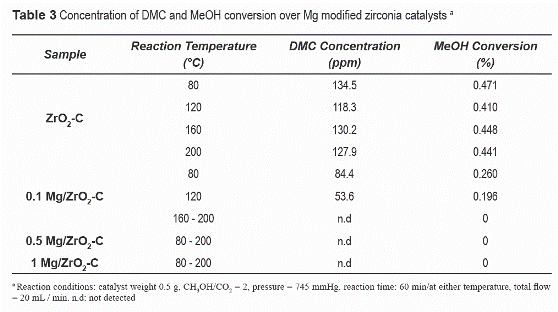
Table 3 shows the concentration of DMC and the conversion of methanol (MeOH) on zirconia materials synthesized with HCl and modified with Mg. As can be observed in table 3, higher conversions are obtained over ZrO2-C than those obtained over Mg-modified samples. In addition, the concentration of DMC remains relatively constant in the temperature range examined. Methanol conversions, although very low (<0.5%), are comparable to those reported by Tomishige et al. [17], where conversions around 0.36% were reached over commercial zirconia in a batch reaction system at considerably higher pressure (5 MPa).
The incorporation of Mg affected methanol conversion. The conversions achieved over Mg- modified materials are 50% lower than those obtained over the unmodified samples (ZrO2-C). In the temperature range evaluated, no methanol conversion was observed over materials containing higher magnesium loadings (0.5% Mg/ZrO2-C and 1% Mg/ZrO2-C). Therefore, although the addition of Mg stabilizes the tetragonal phase of zirconia and increases its CO2 adsorption capacity in the temperature region 100 to 200 °C, methanol conversion to DMC at moderate pressures is not improved.
Conclusions
The type ofhydrolysis catalyst and the incorporation of Mg influence the surface area, crystallinity, basic strength, and CO2 adsorption capacity of zirconia materials. Mg-modified zirconia exhibited higher CO2 adsorption capacity than unmodified zirconia, regardless of the type of hydrolysis catalyst used; however, the strength of basic sites depends on the acid used for hydrolysis, i.e. the tetragonal phase of zirconia and adsorption capacity in the range 100 to 200 °C is obtained when HCl is used. On the other hand, amorphous materials and stronger basic sites with increased CO2 adsorption capacity at temperatures between 600 and 800 °C are obtained for those materials prepared with HNO3 and H2SO4. Mg-modified zirconia exhibited more basic sites in the temperature range where zirconia has been reported to be active for the direct synthesis of dimethyl carbonate (DMC). Nevertheless, it appears that not only basic sites but also, acid sites are required to obtain DMC from methanol and CO2 .
Acknowledgments
The authors would like to thank the Colombian Ministry of Agriculture and Social Development, the University of Antioquia and Química Básica for financial support of this work trough the project: 2007D3608-66. Also, we are also grateful to University of Antioquia for sponsoring our group with the "Sustainability 2009-2010" project.
References
1. Y. Ono. "Dimethyl carbonate for environmentally benign reactions". Catalysis Today. Vol. 35. 1997. pp. 15-25. [ Links ]
2. M. A. Pacheco, C. L. Marshall. "Review of Dimethyl Carbonate (DMC) Manufacture and its Characteristics as a Fuel Additive". Energy Fuel. Vol. 11. 1997. pp. 2-29. [ Links ]
3. D. Delledonne, F. Rivetti, U. Romano. "Developments in the production and application of dimethyl carbonate". Applied Catalysis A: General. Vol. 221. 2001. pp. 241-251. [ Links ]
4. J. Ma, N. Sun, X. Zhang, N. Zhao, F. Xiao, W. Wei, Y. Sun. "A short review of catalysis for CO2 conversion". Catalysis Today. Vol. 148. 2009. pp. 221-231. [ Links ]
5. J. Biana, M. Xiao, S. Wang, X. Wang, Y. Meng. "Highly effective synthesis of dimethyl carbonate from methanol and carbon dioxide using a novel copper- nickel/graphite bimetallic nanocomposite catalyst". Chemical Engineering Journal. Vol. 147. 2009. pp. 287-296. [ Links ]
6. A. Khalid. "Synthesis of dimethyl carbonate (DMC) from methanol and CO2 over Rh-supported catalyst". Catalysis Communications. Vol. 10. 2009. pp. 11271131. [ Links ]
7. K. Tomishige, Y. Ikeda, T. Sakaihori, K. Fujimoto. "Catalytic Properties and Structured of Zirconia Catalysts for Direct Synthesis of DMC from Methanol and Carbon Dioxide". Journal of Catalysis. Vol. 192. 2000. pp. 355-362. [ Links ]
8. K. T. Jung, A. T. Bell. "Effects of Catalyst phase structure on the elementary processes involved in the synthesis of dimethyl carbonate from metanol and carbon dioxide over zirconia". Topics in Catalysis. Vol. 20. 2002. pp. 97-105. [ Links ]
9. K. T. Jung, A. T. Bell. "An in Situ Study of Dimethyl Carbonate Synthesis from Carbon Dioxide and Methanol over Zirconia". Journal of Catalysis. Vol. 204. 2001. pp. 339-347. [ Links ]
10. A. Liu, K. Nyavor, Z. Li, N. O. Egiebor. "Effects of composition and calcination temperatura on morphology and structure of barium modified zirconia nanoparticles". Materials Science and Engineering. Vol. 366. 2004. pp. 66-73. [ Links ]
11. C. Montes de Correa, L. M. Martinez, J. A. Odriozola, M. A. Centeno. "Synthesis and characterization of solgel zirconia supported Pd and Ni catalysts". Catalysis Today. Vol. 107-108. 2005. pp. 800-808. [ Links ]
12. X. Bokhimi, A. Morales, O. Novaro, T. López, R. Gomez. "The effect of hydrolysis initiator on the phase formation in sulfated sol-gel zirconia". Polyhedron. Vol. 19. 2000. pp. 2283-2287. [ Links ]
13. A. Liu, K. Nyavor, R. Ankumah. "Strtuctural and Adsorptive properties of Ba or Mg oxide modified zirconia". Journal of Colloid and Interface Science. Vol. 284. 2005. pp. 66-70. [ Links ]
14. K. T. Jung, A. T. Bell. "The effect of synthesis and pretreatment conditions on the bulk structure and surface properties of zirconia". Journal of Molecular Catalysis A: Chemical. Vol. 163. 2000. pp. 27-42. [ Links ]
15. S. Liu, X. Zhang, J. Li, N. Zhao, W. Wei, Y. Sun. "Preparation and application of stabilized mesoporous MgO-ZrO2 solid base". Catalysis Communications. Vol. 9. 2008. pp. 1527-1532. [ Links ]
16. L. Castro, P. Reyes, C. Montes de Correa. "Synthesis and Characterization of Sol-Gel Cu-ZrO2 and Fe-ZrO2 Catalysts". Journal of Sol-Gel Science and Technology. Vol. 25. 2002. pp. 159-168. [ Links ]
17. K. Tomishige, Y. Ikeda, T. Sakaihori, K. Fujimoto. "A novel method of direct synthesis of dimethyl carbonate from methanol and carbon dioxide catalyzed by zirconia". Catalysis Letters. Vol. 58. 1999. pp. 225-229. [ Links ]
18. Ganapati D. Yadav, Jayesh J. Nair. "Sulfated zirconia and its modified versions as promising catalysts for industrial processes". Microporous and Mesoporous Materials. Vol. 33. 1999. pp. 1-48. [ Links ]
19. L. E. Davies, N. A. Bonini, S. Locatelli, E. E. Gonzo. "Characterization and catalytic activity of zirconium dioxide prepared by sol-gel". Latin American Applied Research. Vol. 35. 2005. pp. 23-28. [ Links ]
(Recibido el 03 de febrero de 2010. Aceptado el 15 de octubre de 2010)
*Autor de correspondencia: teléfono: + 57 + 4 + 219 66 05, fax: + 57 + 4 + 219 65 65, correo electrónico: cmontes@udea.edu.co (C. Montes)