Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Facultad de Ingeniería Universidad de Antioquia
versão impressa ISSN 0120-6230versão On-line ISSN 2422-2844
Rev.fac.ing.univ. Antioquia n.57 Medellín jan./mar. 2011
Effect of the synthesis variables of TiO2 on the photocatalytic activity towards the degradation of water pollutants
Evaluación del efecto de las variables de síntesis de TiO2 sobre su fotoactividad en la degradación de contaminantes del agua
Camilo Andrés Castro López, Sonia Esperanza Reyes Gómez, Aristóbulo Centeno Hurtado, Sonia Azucena Giraldo Duarte*
Centro de Investigaciones en Catálisis (CICAT), Escuela de Ingeniería Química, Universidad Industrial de Santander, Cra. 27 Cl. 9. Bucaramanga. Colombia
Abstract
In this work, TiO2 photocatalysts were synthesized using a conventional sol-gel and hydrothermal synthesis methods with steam pressure treatment. Photocatalysts were characterized by X-ray diffraction (XRD), diffuse reflectance spectra (DRS) and N2 adsorption-desorption. The photoactivity of the samples was analyzed towards the photooxidation of the azo dye Orange II (Or-II) and phenol using different illumination set-ups to compare the activity features of photocatalysts. The effect of the synthesis variables such as the synthesis route, water/alcoxide and alcohol/alcoxide ratios, as well as the alcohol type was analyzed. TiO2 photocatalysts obtained by hydrothermal synthesis have a better photoactivity than the particles synthesized by the chosen sol-gel route, reaching the Or-II degradation photoactivity of the commercial TiO2 P25. On the other hand, the water/alcoxide ratio and alcohol type have a marked effect on the photoactivity of the hydrothermal synthesized TiO2, whereas the alcohol/alcoxide ratio does not have a relevant effect on the Or-II degradation photoactivity.
Keywords: TiO2, photocatalytic oxidation, sol-gel, hydrothermal synthesis, orange II.
Resumen
En este trabajo, se sintetizó TiO2 usando los métodos sol-gel convencional y el hidrotérmico con un tratamiento a presión de vapor. Los fotocatalizadores se caracterizaron por difracción de rayos X (DRX), espectroscopía de reflectancia difusa (ERD) y con base en las isotermas de adsorción-desorciónde N2 se hizo el análisis textural. La fotoactividad de las muestras de TiO2 se evaluó frente a la degradación del colorante Orange II (Or-II) y del fenol bajo irradiación con diferentes sistemas de iluminación. Se analizó el efecto de las variables de síntesis del TiO2 tales como el método de síntesis, las relaciones agua/alcóxido, alcohol/alcóxido y el tipo de alcohol, sobre su fotoactividad. Los resultados muestran que el TiO2 sintetizado por el método hidrotérmico alcanzó la fotoactividad del TiO2 de referencia Degussa P25, mientras que el proceso sol-gel escogido condujo a un TiO2 menos activo. Adicionalmente, se encontró que la relación agua/alcóxido y el tipo de alcohol tienen un marcado efecto sobre la degradación del Or-II mientras que la relación alcohol/alcóxido no presentó un efecto significativo.
Palabras clave: TiO2, oxidación fotocatalítica, síntesis hidrotérmica,síntesis sol-gel, orange II.
Introduction
Titanium dioxide photocatalyst has been proposed as a route to degrade recalcitrant organic pollutants in water and air [1-2], as well as for the deactivation of different bacteria [3-5] and viruses [6]. Based on the semiconductor photochemistry of TiO2, it is possible to generate highly oxidizing radicals after its excitation with ultraviolet light (UV). In this process the irradiation of the TiO2 excites an electron (e-) from the valence band (VB), which is promoted to the conduction band (CB), thus, surpassing the forbidden gap energy or band gap (Eg), leaving a positive charge named hole (h+) on the VB. These photogenerated charges (e- - h+) can simultaneously oxidize the adsorbed H2O or -OH and reduce dissolved O2 to produce oxidizing species such as •OH and O2•- respectively, which are responsible for the oxidation potential of the UV excited TiO2's particles [1].
The sol-gel method has been widely used for the synthesis of TiO2 [1, 5, 7] because it allows to tailor the conditions of synthesis in order to produce crystalline and highly specific surface area particles [7]. Sol-gel involves the hydrolysis of an alcoxide Ti precursor with water in the presence of an alcohol and a subsequent condensation where the Ti-O-Ti network is formed. Therefore, the condensation conducts to the formation of a gel, which is dried at low temperatures. Finally, powders are annealed at 400 - 600 °C to release organic residuals from the TiO2 matrix. More recently, significant variations to the sol-gel process have been adopted to synthesize the TiO2, such as the replacement of the calcination step by hydrothermal treatments under pressure. This pressure treatment promotes the formation of TiO2 particles with high specific surface area, low grain size, and additionally a high cristallinity of the anatase phase [8].
TiO2 has two crystalline forms often applied to photocatalytic reactions. In general, the anatase is considered the crystalline form with the higher photocatalytic activity towards chemical compound oxidation processes. The anatase phase presents an Eg of 3.2 eV allowing wavelengths < 400 nm to produce the charge separation. For rutile phase, the absorption threshold lies at 410 nm. Therefore, TiO2 for solar applications can use only the UV component that is around 3-4% of the solar irradiation hitting the earth's surface. Thus, the crystalline structure and light absorption characteristics of the photocatalyst play a major role in the design of new photoactive materials.
This study focuses on the degradation of the azo dye Or-II in solution under solar-simulated light irradiation. Azo dyes are biorecalcitrant compounds found in common industrial effluents in a wide concentration range [9]. Traditional non-destructive treatment methods such as filtration, reverse osmosis and flocculation have been used to abate this type of pollutant [10]. However, these treatment methods are expensive. In this work, we compared the photoactivity and the characteristics of different TiO2 samples synthesized by the sol-gel and hydrothermal methods towards the photocatalytic degradation of Or-II. The result analyses of the photo- oxidation of Or-II under several light irradiation set-ups such as ultraviolet and solar-simulated light, in addition to the characterization of the materials, led to the understanding of the influence of the synthesis variables affecting the photocatalytic activity of TiO2.
Experimental
Synthesis of TiO2 photocatalysts
Two methods were chosen to synthesize TiO2 photocatalyst: sol-gel and hydrothermal synthesis. TiO2 (SG) was synthesized by the sol-gel route as follows: titanium butoxide (Ti(O- But)4; 97% Aldrich) was added dropwise to isopropanol (Isop-OH; Merck) in a molar ratio Isop-OH/Ti(O-But)4 = 55. Then, HNO3 (65%, Merck) was used as the hydrolysis catalyst, in a molar ratio HNO3/Ti(O-But)4 = 0.173. The amount of water corresponds to a molar ratio H2O/Ti(O-But)4 = 1.5. The formed solution was stirred until gel formation. The gel was aged for 72 h, and immediately dried at 70°C for 4 h. The obtained solid was grounded in a mortar, and the powder calcined at 400 °C for 2 h in a muffle furnace.
TiO2 (HT) was hydrothermally synthesized by hydrolysis of Ti(O-But)4 in aqueous media. In this case, the Ti(O-But)4 was added dropwise to Isop- OH, in a volume ratio Isop-OH/Ti(O-But)4 = 62.5, or with variations from 10 to 62.5, described where needed. In addition, the alcohol was replaced by ethanol and methanol, and also described where needed. Then, a specific volume of water was adjusted to pH = 1.5 using HNO3. This solution was dropwise added to the Ti(O-But)4-Isop-OH solution. The amount of water corresponds to a volume ratio H2O/Ti(O-But)4 = 1 or with variations of 1 to 5 described where needed. The latter suspension was hydrothermally treated with water steam in an autoclave at 120 °C and 1980 KPa during 1 h. Then, the remaining water was extracted by evaporation and continuous stirring at 70 °C. Finally, the obtained powders were grounded in a mortar. TiO2-P25 (P25) from Evonik, previously known as Degussa, was used for comparison purposes.
Photocatalyst characterization
To analyze the phase structure of the photocatalysts, DRX patterns were collected using a D-Max IIIB Rigaku system at room temperature from angles 2θ of 2 to 70°. The diffractometer was operated at 40 kV and 80 mA with a monochromatic Cu Kα radiation.
Specific surface area was determined by the BET method and pore size by the BJH method using a Nova 1300 equipment of Quantachrome.
UV-Vis diffuse reflectance spectra (DRS) were recorded on a PerkinElmer 2034RD lambda 35 UV with an integrating sphere P/NC 6951014 using BaSO4 as a blank reference. The Eg widths of the samples were determined using the Kubelka-Munk phenomenological theory [11]. The DRS can be related to the absorbance by the K/S (where K = absorption and S scattering) ratio using the Kubelka-Munk relationship (F(R∞) (Eq. 1), where the reflectance is noted as R. The reflectance is related to the absorption coefficient α and it is proportional to the absorbance K.
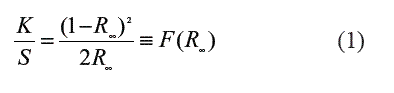
Where R∞ is the ratio of the sample's reflectance over that of the BaSO4 reference. The intercept of the major slope with the wavelength axis gives the value of the sample's Eg if transformed to energy data in eV.
Photocatalytic tests
The photocatalytic degradation of Or-II was carried out with a 20 ppm Or-II aqueous solution with 0.25 g.L-1 of photocatalyst in borosilicate glass reactors of 50 mL of volume capacity. Phenol degradation was made using a 0.1 mmol.L-1 solution and a TiO2 suspension of 1 g.L-1. This suspension was stirred 1 h under darkness prior to illumination. The reactors were irradiated under three different illumination systems: (I) under 400 W.m-2 of solar- simulated light using a Suntest system CPS+ from ATLAS. This lamp has a spectral distribution with about 0.5% of emitted photons at wavelengths shorter than 300 nm (UV-C range), and about 4% between 300 and 400 nm (UV-B and UV-A ranges). The distribution of the photons emitted between 400 and 800 nm follows the solar spectrum (II) under 38 W.m-2 of ultraviolet light using a set of 5 BLB Phillips lamps (TLD 18 W) with a spectrum emission between 330 and 400 nm, or (III) under 60 W.m-2 visible light using a set of 5 Blue Phillips lamps (TLD 18 W) with emission wavelengths between 400 and 500 nm. The radiant flux was monitored with a Kipp & Zonen (CM3) power meter (Omni instruments Ltd., Dundee, UK). Samples were taken every 15 min for 1 h and centrifuged at 3000 rpm for 15 min. The concentration of Or-II in the supernatant was determined with a HP 8453 UV-Vis spectrophotometer at the Or-II's maximum wavelength absorption of 486 nm. Phenol oxidation was followed by sampling 1 mL every 15 min and filtrating with a 0.45 μm pore membrane. Phenol detection was made using a HPLC Hewlett- Packard series 1100 equipment with a reverse phase Spherisorb silica column (Macherey-Nageland) and a diode array detector, usig a wavelenght of 220 nm. As mobile phase a mixture of acetonitrile and water in a volume ratio of 60/40 was used. Blank experiments were carried out to determine possible degradation by photolysis. The total organic carbon (TOC) concentration was followed in a Shimadzu 500 system with autosampling. The reported data as X/Xο, where X = C for Or-II concentration in mg/L or X = TOC concentration in mg/L, denotes the ratio of the concentration at any given time to the initial concentration. The concentration reached after 1 h of stirring under darkness was used as the initial value of the concentration (Xο) in the photodegradation analysis.
Results and discussion
Comparative synthesis routes of TiO2
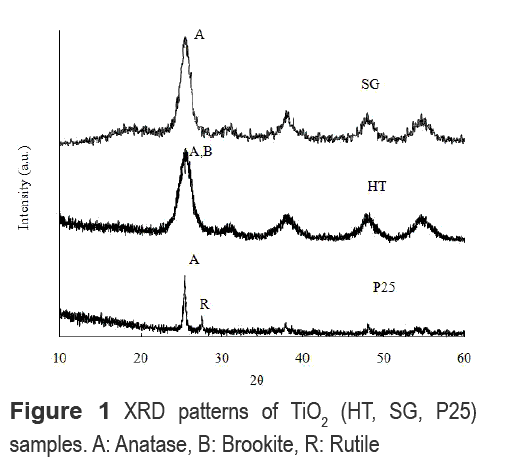
From XRD analysis shown in figure 1 it is observed that the phase with major presence in the obtained TiO2 particles is anatase, which has a characteristic peak at 2θ = 25.2°. In HT, the main peak of anatase overlaps with the diffraction peaks of brookite phase at 2θ= 25.34° and 25.68°, which is a characteristic when both phases coexist in TiO2 structure [12]. In SG, the anatase phase has a high crystallinity. This high crystallinity of anatase phase is related to a high photocatalytic activity of the TiO2 [13].
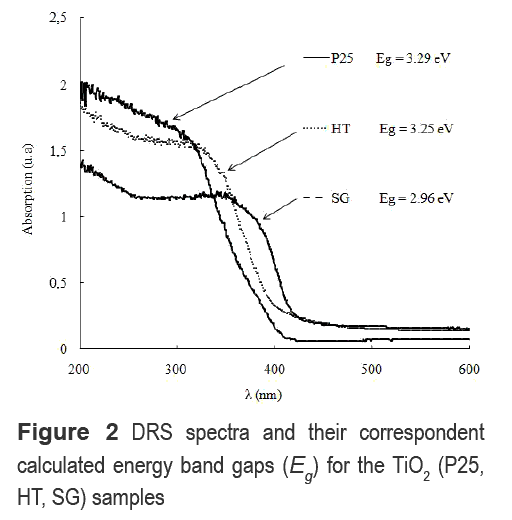
DRS spectra of the TiO2 samples and their correspondent energy band gaps (Eg) obtained by the Kubelka-Munk theory are shown in figure 2. P25 shows the highest absorption in the UV region of the spectrum (200-360 nm). Samples obtained by the sol-gel process exhibit the lowest Eg while HT and P25 have similar values.
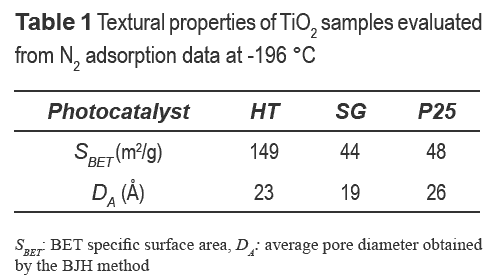
Textural properties, the specific surface area (SBET), and average pore diameter (DA) of photocatalysts are shown in table 1. Hydrothermal synthesis led to TiO2 particles (HT) with higher specific surface area and similar average pore diameter than SG and P25.
The chosen sol-gel and the hydrothermal method led to the synthesis of TiO2 samples with different crystalline structures, specific surface areas and different light absorption capacities. Thus, it is expected that the synthesis method influence the photoactivity of TiO2 since it alters remarkably its physical and semiconductor features.
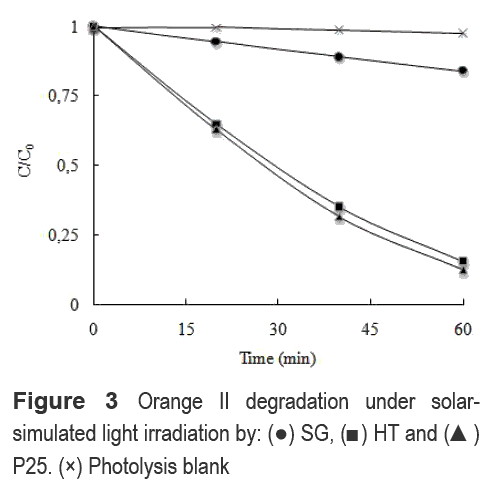
Figure 3 shows the photocatalytic degradation of Or-II by the TiO2 photocatalysts under solar- simulated light irradiation. The SG sample shows the lowest performance in the photodegradation of Or-II despite of its high cristallinity. No correlation between the crystallinity of phases in SG and its photoactivity was found.
However, it is possible to suggest that the coexistence of two phases in TiO2 may explain the higher activity of P25 and HT samples when compared to the one of SG with anatase phase solely. The coexistence of TiO2 phases has been suggested to reduce the recombination of the e-- h+ pair [12, 14]. The anatase phase, with a Eg of 3.2 eV, is known as the most photoactive TiO2 phase. Nevertheless, an anatase to rutile phase (Eg of 3.0 eV) ratio of 3:1 of the commercial TiO2 P-25 shows the best photocatalytic activity [14]. Such a difference in the Eg of the anatase and rutile phases serves as an energy gradient, which conducts the e- from rutile to anatase, thus, avoiding the recombination. Similarly, a mixed phase TiO2, constituted of anatase and brookite phases, has a higher performance towards the degradation of 2-chlorophenol than pure anatase TiO2 [12]. Then, an increase in photoactivity seems to be related to the coexistence of anatase and rutile [14] or brookite in TiO2 [15].
Moreover, the light absorption capacity of the SG sample (Figure 2) suggests its activity under visible light illumination. However, no significant activity was found under visible light illumination with any of the samples.
In addition, the initial velocities of phenol degradation, that were determined during the first 30 min. of reaction, by HT, P25 and SG are 3.3x10-3, 4.5x10-3, and 0.2x10-3 μmol.L-1. min-1, respectively. These results support that the HT sample has a similar photo-oxidation activity than the P25 sample; meanwhile, the SG sample maintains its low performance. The difference in activity for HT and P25 samples may be due to different oxidation pathways for Or-II and phenol. Or-II may be oxidized by HT due to a surface-hole-mode oxidation as we have already proposed [16]. Phenol, in contrast, is oxidized in the homogenous phase by action of •OH radicals due to its low adsorption on the P25's surface as proposed by Sobczynski et al [17].
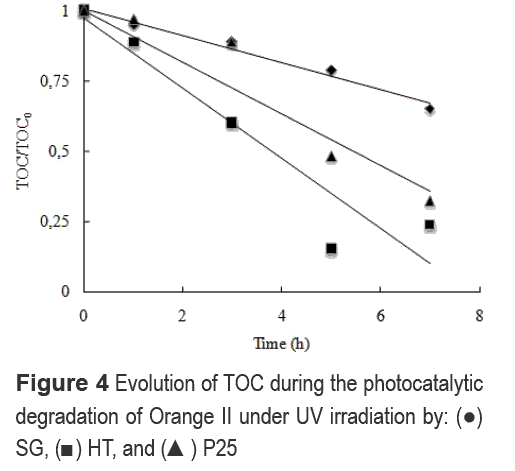
Figure 4 shows the evolution of the TOC concentration during the photodegradation of Or-II under UV irradiation. Results evidence again the poor photoactivity of the SG sample. This implies that there is no correlation between the light absorption capacity and the activity under visible light irradiation for SG. It is possible to suggest the reduced activity of the SG sample is due to the removal of adsorbed species such as -OH, which is possibly due to the uncontrolled conditions during the drying and calcinations steps of the synthesis. Hart et al [18] suggested that the increase in the drying temperature reduces the presence of adsorbed species such as -OH, which indeed can inhibit the recombination by trapping the h+, and thus, forming the •OH radical. Hence, during the synthesis procedure, we may have reduced the surface reactivity by promoting the desorption of -OH, and thus, decreased the photoactivity of the SG sample.
As seen in Figure 4 under UV irradiation the overall mineralization caused in the system by HT is higher than P25 even with a similar reduced concentration of Or-II followed by absorption at 486 nm (Fig. 3). This implies a higher oxidation potential of the HT sample which is possibly due to the higher surface area of HT particles that may promote a higher adsorption of Or-II, and thus, the possibility for the molecule to be oxidized is increased.
Once evidenced that the hydrothermal synthesis produces photoactive particles, the effect of hydrothermal synthesis variables such as water/ alcoxide and alcohol/alcoxide ratios and the alcohol type used as cosolvent for the hydrolysis of the Ti precursor were analyzed towards the Or- II photocatalytic degradation.
Effect of the TiO2 liquid phase synthesis variables on its photocatalytic activity
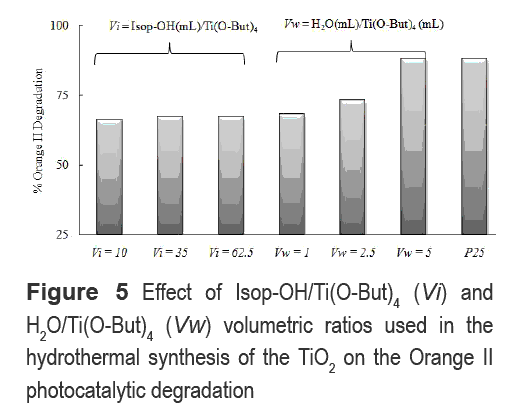
Figure 5 shows the photocatalytic degradation of Or-II using TiO2 samples synthesized by the hydrothermal method using different volumetric ratios of Isop-OH/Ti(O-But)4 (Vi) and H2O/ Ti(O- But)4 (Vw).
The variation of the alcohol amount used in the hydrothermal synthesis analyzed here (Vi = 10-62.5) did not cause a relevant change on the photocatalytic activity of the HT sample. On the contrary, Xu et al [8] reported that the increase in the alcohol concentration during the synthesis reduces the photoactivity due to an increased amount of amorphous in the TiO2 structure. Nevertheless, the increase in the VH2O, as shown in figure 4, increases the photoactivity. Possibly the decrease of Isop-OH concentration, caused by the increase in the quantity of water, may reduce the negative effect over the hydrolysis reaction due to the presence of Isop-OH [8]. Therefore, particles with a high crystallinity are produced, which are expected to have a better photoactivity than amorphous particles.
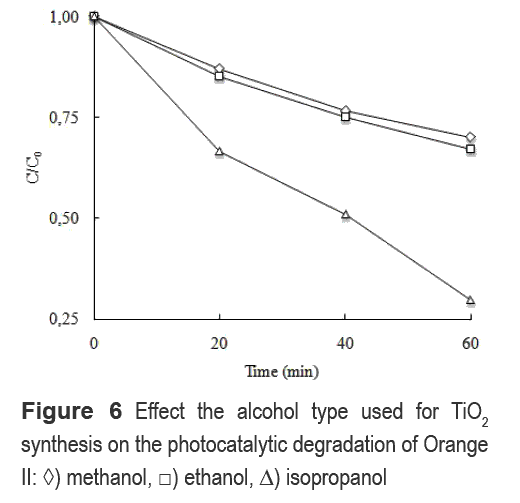
On the other hand, the effect of the alcohol type used during the hydrothermal synthesis is shown in figure 6. The X-ray difractrograms of the samples (not shown here) are very similar to the HT sample (Fig. 1) and thus they are expected to have the same crystallite sizes. However, the photocatalytic activity is different.
Possibly, the reduction of photoactivity caused by the use of short linear chain alcohols, such as, methanol and ethanol may be due to the tendency of these to form hydrogen bonds with the surface hydroxyl groups reducing the amount of oxidation sites on the TiO2 surface [19]. Moreover, the exchange of radicals between the Ti alcoxide and the alcohol may be affected by the use of linear alcohols such as methanol and ethanol, or branched type alcohols such as isopropanol. Therefore, the use of isopropanol may promote the formation of a more defective particle than linear alcohols [7], and thus, the surface area available for photon adsorption by TiO2 is expected to increase, promoting the possibility to separate the electron and hole on the surface of the photocatalyst. The SBET for the HT synthesized samples with methanol, ethanol and isopropanol are 84, 95 and 160 m2/g respectively. These results confirm that the use of branched alcohols increase the specific surface area during the hydrothermal synthesis of TiO2.
Conclusions
Crystalline TiO2 powders with major presence of anatase phase in their structure were synthesized by the sol-gel and hydrothermal synthesis methods using steam pressure. The synthesis route influence the activity of the TiO2, possibly by the different physical features given to synthesized samples by the different synthesis conditions. The hydrothermal synthesis route led to TiO2 particles with a higher light absorption capacity on the UV region, a higher specific surface area and a better Or-II photo- oxidation activity under UV and solar-simulated light irradiation than the sample synthesized by the sol-gel route. In contrast, the photocatalytic degradation of phenol revealed differences in photoactivity of the samples, which are possibly due to different oxidation modes promoted on the TiO2's surface obtained by the hydrothermal synthesis, or in the homogeneous phase by •OH radicals produced by the commercial TiO2 P25.
The water/alcoxide ratio and alcohol type used for the synthesis have a marked effect on the photoactivity of the HT sample, while the alcohol/alcoxide ratio does not have a significant effect on the Or-II degradation photoactivity. The photoactivity increase due to the increase of the water/alcoxide ratio is possibly due to alcohol dilution, which diminishes its negative effect towards the hydrolysis of the alcoxide. Moreover, the use of branched type alcohols, such as, isopropanol increases the activity of the photocatalyst, possibly due to the increase in the available area for UV light absorption.
Acknowledgments
This work was financially supported by COLCIENCIAS and SENA (Project code: 1102341-19419). Financial support by the named government entities and UIS for the PhD study of Camilo Castro is gratefully acknowledged. Special acknowledgements to: Institute of Chemical Sciences and Engineering of the EPFL, Switzerland, for the HPLC and TOC analysis.
References
1. O. Carp, C. L. Huisman, A. Reller. "Photoinduced reactivity of titanium dioxide". Prog. Solid State Chem. Vol. 32. 2004. pp. 33-177. [ Links ]
2. J. M. Herrman. "Heterogeneous photocatalysis: fundamentals and applications to the removal of various types of aqueous pollutants". Catal. Today. Vol. 53. 1999. pp. 115-129. [ Links ]
3. D. Gumy, C. Morais, P. Bowen, C. Pulgarín, S. Giraldo, R. Hajdu, J. Kiwi. "Catalytic activity of commercial TiO2 powders for the abatement of the bacteria (E. coli) under solar simulated light: Influence of the isoelectric point". Appl. Catal. B. Vol. 63. 2006. pp. 76-84. [ Links ]
4. J. Blanco Galvez, P. Fernández Ibáñez, S. Malato Rodríguez. "Solar Photocatalytic Detoxification and Disinfection of Water: Recent Overview". J. Sol. Energy Eng. Vol. 129. 2007. pp. 4-15. [ Links ]
5. C. Castro, A. Arámbula, A. Centeno, S. A. Giraldo. "Degradación Heliofotocatalítica de Escherichia coli en sistemas tipo Desinfección SODIS, con Dióxido de Titanio modificado". Inf. Tecnol. Vol. 20. 2009. pp. 29-36. [ Links ]
6. L. Zan, W. Fa, T. Peng, Z. Gong. "Photocatalysis effect of nanometer TiO2 and TiO2-coated ceramic plate on Hepatitis B". J. Photochem. Photobiol. B. Vol. 86. 2007. pp. 165-169. [ Links ]
7. I. A. Montoya, T. Viveros, J. M. Dominguez, L. A. Canales, I. Schifter. "On the effects of the sol-gel synthesis parameters on textural and structural properties of TiO2". Catal. Lett. Vol. 15. 1992. pp. 207-217. [ Links ]
8. S. Y. Xu, W. Zheng, W. Liu. "Enhanced photocatalytic activity of supported TiO2: dispersing effect of SiO2". J. Photochem. Photobiol. A. Vol. 122. 1999. pp. 57-60. [ Links ]
9. R. Anliker. "Ecotoxicology of dyestuffs - a joint effort by industry". J. Ecotox. Environ. Saf. Vol. 3. 1979. pp. 59-74. [ Links ]
10. M. R. Hoffman, S. T. Martin, W. Choi, D. W. Bahnemann. "Environmental applications of semiconductor photocatalysis". Chem. Rev. Vol. 95. 1995. pp. 69-96. [ Links ]
11. P. Kubelka, F. Munk. "Ein Beitrag zur Optik der Farbanstriche". Z. Tech. Phys. Vol. 12. 1931. pp. 593-601. [ Links ]
12. S. Ardizzone, C. Bianchi, G. Cappelletti, S. Gialanella, C. Pirola, V. Ragaini. "Tailored Anatase/Brookite Nanocrsytalline TiO2. The optimal Particle Features for Liquid and Gas-Phase Photocatalytic Reactions". J. Phys. Chem. C. Vol. 111. 2007. pp. 13222-13231. [ Links ]
13. K. Y. Jung, S. B. Park. "Anatase-phase titania: preparation by embedding silica and photocatalytic activity for the decomposition of trichloroethylene". J. Photochem. Photobiol. A. Vol. 127. 1999. pp. 117-122. [ Links ]
14. D. C. Hurum, A. G. Agrios, K. A. Gray, T. Rajh, M. C. Thurnauer. "Explaining the enhanced photocatalytic activity of Degussa P-25 mixed phase oxide TiO2 using EPR". J. Phys. Chem. Vol. 107. 2003. pp. 4545-4549. [ Links ]
15. A. Di Paola, G. Cufalo, M. Addamo, M. Bellardita, R. Campostrini, M. Ischia, R. Ceccato, L. Palmisano. "Photocatalytic activity of nanocrystalline TiO2 (brookite, rutile, and brookite-based) powders prepared by thermohydrolysis of TiCl4 in aqueous chloride solutions". Colloids Surf. A: Physicochem. Eng. Aspects. Vol. 317. 200S. pp. 366-376. [ Links ]
16. C. A. Castro-López. A. Centeno, S. A. Giraldo. "Fe-modified TiO2 photocatalyst for the oxidative degradation of recalcitrant water contaminants". Catal. Today. Vol. 157. 2010. pp. 119-124. [ Links ]
17. A. Sobczynski, L. Duczman, W. Zmudzinsky. "Phenol destruction by photocatalysis on TiO2: an attempt to solve the reaction mechanism". J. Mol. Catal. A. Vol. 213. 2004. pp. 225-230. [ Links ]
18. J. N. Hart, L. Bourgeois, R. Cervini, Y. B. Cheng, G. P. Simon, L. Spiccia. "Low temperature crystallization behavior of TiO2 derived from a sol-gel process". J. Sol-Gel Sci. Techn. Vol. 42. 2007. pp. 107-117. [ Links ]
19. E. Alonso, I. Montequi, M. J. Cocero. "Effect of synthesis conditions on photocatalytic activity of TiO2 powders synthesized in supercritical CO2". J. Supercritic. Fluids. Vol. 49. 2009. pp. 233-238. [ Links ]
(Recibido el 03 de febrero de 2010. Aceptado el 15 de octubre de 2010)
*Autor de correspondencia: teléfono: + 57 + 7 + 634 47 46, fax: + 57 + 7 + 645 96 47, correo electrónico: sgiraldo@uis.edu.co (Sonia. A. Giraldo)