Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230On-line version ISSN 2422-2844
Rev.fac.ing.univ. Antioquia no.60 Medellín Oct./Dec. 2011
A novel aerobic-anoxic biological filter for nitrogen removal from UASB effluent using biogas compounds as electron donors for denitrification
Nueva configuración de filtro biológico aerobio- anóxico para la remoción de nitrógeno del efluente de un reactor UASB usando biogás como donador de electrones para la desnitrificación
Jenny Rodríguez Victoria1*, Eugenio Foresti2
1EIDENAR. Universidad del Valle, Ciudad Universitaria Meléndez. Calle 13 N° 100-00. Cali, Colombia.
2Departamento de Hidráulica e Saneamento. Escola de Engenharia de Sao Carlos. Universidade de Sao Paulo. Av. Trabalhador Sao-carlense, 400. Sao Carlos, Brasil.
Abstract
The performance of a new trickling filter (TF) configuration composed of an upper compartment for nitrification and a lower compartment for denitrification of effluent from a UASB reactor treating domestic sewage was evaluated. The TF was packed with new plastic material characterized by its durability and high percentage of void spaces. The feasibility of using the reduced compounds present in the biogas produced by a UASB reactor as electron donor for denitrification was also evaluated. Efficient nitrification and denitrification was achieved for the mean hydraulic (5.6 m3 m-2 d-1), organic (0.26 kg COD m-3 d-1) and ammonia-N (0.08 kg m-3 d-1) loading rates applied, resulting in ammonia-N removal ranging from 60 to 74%. The final effluent presented ammonia-N lower than 13 mg L-1. Despite the presence of dissolved oxygen (DO) in the denitrification compartment, its performance was considered quite satisfactory and final nitrate concentrations were lower than 10 mg L-1. The results indicate that methane was the main electron donor used for denitrification. Additionally, denitrification can probably be improved by avoiding high DO concentration in the denitrification compartment and by enhancing biogas transfer in the anoxic zone.
Keywords: nitrification, denitrification, nitrogen, methane, biological filter, UASB.
Resumen
El presente estudio tuvo como objetivo evaluar una nueva configuración de filtro biológico, aerobio, para obtener la nitrificación y desnitrificación del efluente de un reactor UASB que trata agua residual doméstica. El filtro biológico estuvo compuesto por dos compartimientos, uno superior aerobio nitrificante simulando un filtro percolador y uno inferior anóxico desnitrificante con medio de soporte sumergido. Adicionalmente, fue evaluada la factibilidad de usar el biogás producido en el reactor UASB como donador de electrones para la desnitrificación. Para una carga hidráulica aplicada de 5.6 m3 m-2 d-1, una carga orgánica aplicada de 0.26 kg DQO m-3 d-1 y una carga aplicada de nitrógeno amoniacal de 0.08 kg m-3 d-1 se obtuvo una transformación del nitrógeno amoniacal entre el 60 y 74%, con concentraciones efluentes menores de 13 mg L-1. A pesar de la presencia de oxígeno disuelto en el compartimiento de desnitrificación, se alcanzaron concentraciones de nitrato efluente menores de 10 mg L-1. Los resultados obtenidos indican que el metano presente en el biogás, fue el principal donador de electrones para la desnitrificación.
Palabras clave: nitrificación, desnitrificación, nitrógeno, metano, filtro biológico, UASB.
Introduction
Given environmental, cultural, and economic conditions in Latin-American countries, waste-water treatment systems have to be functionally simple; the cost-benefit ratio has to be high and; the applied technologies have to be appropriate to local realities. At the moment, none of the simple and low-cost technologies available satisfy all of the dispositions imposed by environmental protection requirements. Even so, despite anaerobic technology in its diverse forms not representing the only nor the best technology for environmental and health protection, it has been considered viable to be implemented in many cities.
The environmental conditions in developing countries, besides the low costs and efficient removal of biodegradable matter of anaerobic reactors, make the application of the anaerobic technology very favorable under the perspective of sustainable development. However, this technology produces an effluent with relatively high concentrations of suspended solids and residual organic material. It also shows poor capacity to remove nitrogen and pathogens. Therefore a post-treatment step is usually required.
Regarding biological nutrient removal from wastewater, various system configurations can be adopted. Recently, a number of new processes and reactor configurations were developed. Immobilized biomass reactors have been increasingly used for nitrogen removal [1-6], achieving high performance and stability because they can efficiently retain the biomass inside them allowing for the operation at high cellular retention times. This condition favors nitrification and denitrification processes to occur in a sole unit. As biomass immobilization normally results in high-cell concentrations, the volumetric efficiency is greatly increased. This can lead to relatively small reactors, and may afford protection from toxic shocks and adverse temperatures [7].
Trickling filters (TF) have been used successfully for BOD removal only, for combined BOD removal and nitrification and for tertiary nitrification after secondary treatment. The first investigations with tertiary nitrification indicated that the filters filled with plastic support are stable reactors able to produce a high effluent quality even under adverse conditions [8-10]. Its simple operation along with its low operation and maintenance costs were also demonstrated. Four operating variables: temperature, influent ammonia concentration, hydraulic loading, and recirculation, are considered important for nitrification in trickling filters. Subsequent studies revealed that nitrification is often diffusion-limited and it will depend on the ammonia loading, oxygen availability, temperature and support media [11-14].
Some experiences have demonstrated that the combination of BOD and ammonia removal in nitrifying trickling filters (NTF), besides being possible, is frequent [15, 16]. The high cellular retention time and oxygen concentration present in NTF create appropriate conditions for heterotrophic population growth and accumulation [17].
Both BOD removal and nitrification can be obtained in TFs operated at low organic loading rates [18]. Due to competition between heterotrophic and autotrophic bacteria, significant nitrification occurs only after the organic matter concentration is considerably reduced. From different experiments, it can be concluded that nitrification takes place mainly at the bottom portion of the filter. In this region, BOD should be under 30 mg L-1 for nitrification initiation and under 15 mg L-1 for complete nitrification [15, 16].
A number of investigations have shown that trickling filters can perform aerobic, anoxic, and anaerobic processes depending on the biofilm characteristics (thickness and activity), availability of oxygen, presence of nitrate, and concentration and nature of the electron donors [15,19-22]. For these reasons, denitrification could be obtained in TFs when their effluents are recycled back to existing upstream of low-loaded carbonaceous TFs.
The use of TF for the post-treatment of anaerobic reactor effluents is increasingly accepted as both a technically and economically feasible alternative, especially for removal of the remaining COD and suspended solids [23]. However, the applicability of TFs for nitrogen removal of anaerobic effluents has not been well studied so far.
Nitrogen removal from anaerobic effluents in TFs proceeds via nitrification followed by denitrification. During the nitrification step, however, most of the effluent organic matter that could be used for denitrification is oxidized. Therefore, supplemental electron donors are required for denitrification to proceed. It should be noted that the need for the supplementary addition of an external carbon source for denitrification makes it inconvenient from the sustainability point of view. Recently, search for electron donors produced during the wastewater treatment processes has deserved special attention from researchers aiming to lower the costs of denitrification. The literature suggests that methane and reduced sulfur compounds could be possible and interesting alternative electron donors [24-28]. Moreover, anaerobic treatment plants produce CH4 and H2S containing biogas. Consequently, it is expected that anaerobic technology based systems can produce low-cost and efficient electron donors readily useable for denitrification.
Therefore, the UASB-TF combination can present relevant advantages relative to operation simplicity and low costs if the biogas produced in the UASB reactor can be used for denitrification. Such a concept implies modifications in TF configurations to incorporate a denitrification compartment equipped with a biogas distribution system below the nitrification compartment.
This paper presents and discusses data on the performance of a novel, two-compartment integrated aerobic-anoxic TF for the post treatment of effluents from a UASB reactor treating domestic sewage. Special emphasis is given to nitrogen removal in the denitrification compartment fed with biogas as electron donor source.
Experimental apparatus and methods
Reactor description
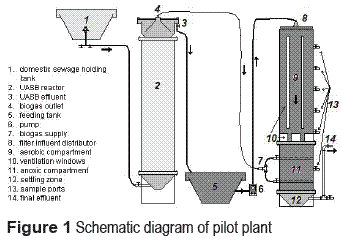
The pilot scale treatment plant consisted of two sequentially disposed units. The first unit was a UASB reactor with a useful volume of 0.2 m3. The effluent of the UASB reactor was treated in the second unit, the aerobic-anoxic trickling filter (AATF). A schematic diagram of the pilot plant employed in this study is shown in figure 1.
The filter was constructed in PVC with a diameter of 0.35 m and total height of 2.70 m. The AATF was composed of two vertically disposed compartments. The upper aerobic compartment had a trickling filter configuration for removal of residual COD and nitrification with a useful volume of 200 L. In order to improve natural aeration, vertical ventilation windows were provided in the external wall. The lower compartment (60 L) was meant to be operated under anoxic conditions for denitrification. For this reason, it was submerged. Five sampling ports were located along the upper compartment height (SU, 1F1, 2F1, 3F1 and 4F1); whereas, the lower compartment had three sampling ports (F1, 6F2 and F2). Biogas employed as electron donor was supplied by two superposed manifolds located inside the anoxic compartment and connected to the UASB reactor biogas outlet line.
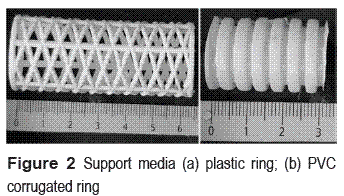
The support media were novel plastic rings commercially known as "rulo" (3.3 cm in diameter, 6.4 cm in length, void ratio 95% and specific surface area 143 m2 m-3) obtained from local suppliers. Additional to the "rulo", PVC corrugated rings were used in the anoxic compartment (2.2 cm in diameter, 3.0 cm in length, void ratio 94% and specific surface area 135 m2 m-3). Pictures of the support media are shown in figure 2.
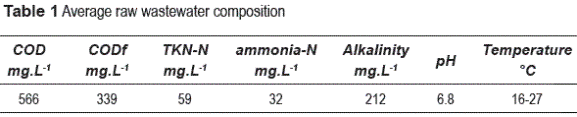
After the star-up period, the AATF was continuously operated at the flow rate of 0.54 m3 d-1, corresponding to the hydraulic loading rate of 5.6 m3 m-2 d-1. Organic loading rate ranged from 0.22 - 1.2 kg COD m-3 d-1, and ammonia-N loading rate ranged from 0.062 - 0.104 kg m-3 d-1, according to variations of COD and ammonia-N in the UASB reactor effluent. Due to operating problems along the first operation Stage, the pilot UASB operation was interrupted and the filter started to be fed with the effluent from a full-scale UASB reactor treating domestic wastewater. The full-scale UASB reactor also provided the biogas for denitrification. The biogas was constantly flushed with a mean flow of 1.7 L min-1 at the mean composition of 376 mg L-1 of CH4, 112 mg L-1 of CO2, and 0.493 mg L-1 of H2S. The average composition of the UASB influent is summarized in table 1.
Analytical methods
Monitoring consisted in collecting samples once or twice a week at different points of the reactor. The parameters analyzed were: temperature, pH, COD, ammonia-N, nitrate, nitrite, and sulfate. Analytical determinations were according to procedures recommended by the Standard Methods for the Examination of Water and Wastewater [29]. Composition of biogas was determined by gas chromatography by using a thermal conductivity detector. Aiming at verifying if the increase of ammonia-N in the denitrifying compartment was due to dissimilatory metabolism, a denitrification assay was carried out by using a modification of the method described by [30]. Additionally, the thickness of the biofilm along the height of the filter was regularly examined under a microscope equipped with an ocular micrometer.
Results and discussion
Biofilm development
A fast colonization of microorganisms was observed mainly inside the support media. During colonization, biomass growth was patchy. Colonization began with the emergence of a gelatinous substance on the whole surface of the support media. After the biofilm reached a given thickness (0.5 mm approx.), the gelatinous substance practically disappeared. The biomass attached on the support media suffered stratification along the height of the filter; with the absolute predominance of heterotrophic populations in the upper part of filter. This fact can be attributed to the high organic loads applied due to the operating problems occurring with the UASB reactor, which resulted in high COD concentrations in its effluent. According to microscopy observations (data not shown), such as the organic matter being consumed, the nitrifying population was increasing along the filter.
Organic matter removal
The analysis of the COD results was divided into two stages regarding the application of different organic loads. During Stage 1, the filter received the effluent from the pilot UASB reactor. This period can be characterized by the application of higher organic loading rates due to operating problems in the pilot UASB reactor. As a consequence, the filter performance was also unstable and low COD removal efficiencies (< 50%) were observed. Even so, filter effluent COD was approximately constant (< 20 mg L-1) by the end of Stage 1, independently on the applied organic loading rate. This result confirms the capacity of AATF to efficiently remove high organic loads (COD removal ~ 90%). It also indicates a progressive development of the biomass throughout a selective process leading the heterotrophic population to settle down mainly until the middle of the aerobic compartment. Stage 2 corresponded to the use of the full-scale UASB effluent, whose operation was stable and COD removal efficiencies were higher than those observed when the effluent of the pilot UASB reactor was used to feed the TF. Organic loading rates were lower than those applied during Stage 1.
Nitrification-aerobic compartment
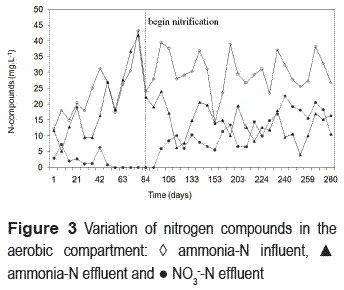
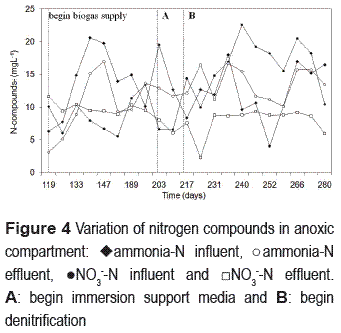
Nitrification was clearly manifested on the 98th day of operation (figure 3).
At the beginning of nitrification, effluent ammonia concentrations were mainly related to the influent values. Thereafter, such dependence was reduced and better efficiencies were obtained, indicating the nitrifying biomass was growing and attaining stability with time. By the end of the operation period, the average effluent ammonia-N concentration was 13 mg L-1, the average effluent NO3-N concentration was 12 mg L-1 and the average nitrification efficiency was 60%. Ammonia concentrations are expected to decrease along the reactor height in nitrification TFs. In this study, a slight increase of ammonia concentration was observed on some occasions. This may have been caused by nitrogen compound releases from cellular lyses, probably due to the high amount of biomass accumulated at the bottom of the aerobic compartment. A similar trend was found by [31]. Despite the increase of ammonia concentration, a decrease of effluent nitrate concentrations was observed. Such a denitrification process near the bottom of the nitrifying compartment can be attributed to the existence of anoxic microenvironments generated by the accumulation of biomass in this region.
DO concentrations along the aerobic compartment ranged from 3 to 5 mg L-1; thus, confirming the adequacy of adopting ventilation windows in the external filter wall. Significant decreases of DO inside the reactor were not observed. Therefore, the nitrification process was not limited by oxygen availability as the high void space of support media allowed for an efficient air supply inside the filter.
Denitrification-anoxic compartment
Once nitrification was obtained, the biogas supply was started, aiming at establishing the denitrification process. Before being submerged, the denitrifying compartment was not free from DO (4 mg L-1). Additionally, nitrifying biomass was also washed out from the aerobic compartment and retained in the denitrifying compartment. Initially, this fact favored the nitrification and limited the denitrification. In fact, the characteristics of the support media propitiated favorable conditions for the free circulation of air and biogas. Such a condition did not allow the suitable contact between biogas and the denitrifying bacteria to occur, affecting the availability of electron donor (biogas) for denitrification. To improve environmental conditions for denitrification, the support media was submerged (204th day). The objective was to offer a resistance to the rapid biogas ascension and mixture with the air, thus allowing a better contact between the biogas compounds and the denitrifying bacteria. As shown in figure 4, the denitrification process was improved after the support media was submerged (217th day). The average effluent NO3-N concentration dropped to 8 mg L-1 and the average denitrification efficiency was 52%.
Simultaneous to denitrification improvement, an increase of effluent ammonia-N over the influent concentration was observed. In order to understand this phenomenon, a test of dissimilatory nitrate reduction to ammonium in denitrifying biomass was performed as described by [30]. The production of ammonium was not observed, indicating that dissimilatory nitrate reduction was not taking place inside the reactor. The other possible reason for the increase in ammonia-N concentration would be the endogenous metabolism of the biomass accumulated at the bottom of the nitrification- aerobic compartment. Aiming at avoiding the production of N compounds via cellular lyses, the sludge accumulated at the bottom of the compartment started to be removed once a week.
There are two compounds in the biogas that are potentially used as electron donor for denitrification: CH4 and H2S. The effective use of such in denitrification depends on environmental conditions as it is the presence or absence of DO. In the present study, the biogas was probably the sole electron source for denitrification since organic matter availability from wastewater was very low (COD < 20 mg L-1). Additionally, no significant variation of COD concentrations between influent and effluent was observed as expected if COD was used as electron donor.
Considering that DO was also present in the denitrification compartment (1 mg L-1), it can be assumed that this fact favored the oxidation of methane present in the biogas to intermediate organic compounds, which were then used for denitrification. This effect was observed by [24] in laboratory experiments. Nevertheless, with an excess of DO concentration complete oxidation of methane to CO2 can occur resulting in the consumption of significant fraction of methane limiting its use as electron donor for denitrification.
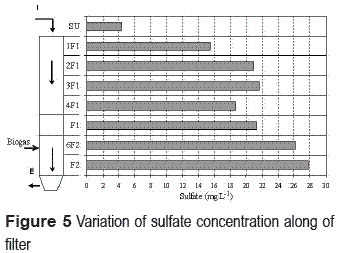
On the other hand, denitrification occurred concomitantly with the increase of sulfate concentration. Due to the presence of DO in the compartment, it is suggested that sulfate was produced by full oxidation of H2S from biogas and probably also by its use for denitrification. In figure 5 is observed as the sulfate has its highest values in the closest ports of supply biogas, with an increase in port F2 and a tendency to decrease as the rises by AATF biogas. The highest values of sulfate in F2 can be the result partly of sulfate produced in the top spots that the downflow of wastewater, have led to an increase in its concentration of ports below. The lowest values of sulfate are attributed to the decrease in availability biogas. H2S was being partly oxidized into its upward, due to high DO concentration present between ports F1 and 1F1 and partly released to the outside of the AATF through ventilation windows.
As reported in the literature [32], there are indications that denitrification occurs more easily by using sulfur compounds than methane. In contrast, the low concentrations of H2S in the biogas could have limited its utilization as electron donor for denitrification.
In spite of the fact that it was not possible to demonstrate that the H2S present in the biogas was fully used for denitrification, H2S was transformed inside the filter and removed from the biogas. This fact demonstrated an additional ability of the AATF that can be used for the treatment of the biogas produced in anaerobic reactors.
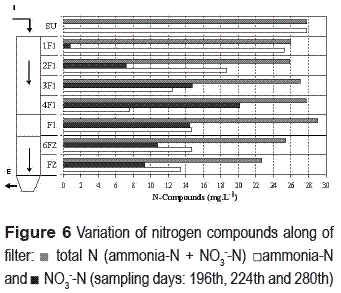
The evolution of the nitrogen species along the reactor, from ammonia-N to nitrate in the nitrifying compartment and the performance of the denitrifying compartment is shown in figure 6. In port F1, a significant increase in ammonia-N concentration can be observed due to biomass accumulation in the interface between the compartments, as well as, the persistence of this compound at a stable concentration from this port onwards. As expected, NO3-N reduction by denitrification occurred steadily along the lower compartment, but the process was probably limited by the electron donor availability.
From the data obtained in this research, aerobic/anoxic TFs can be applied for the post-treatment of effluents from anaerobic reactors. The unit removed significant fractions of the soluble COD and nitrogen. Even considering the limitations observed, the effluent produced attains the requirements of organic matter and nitrogen compound concentrations for discharge in superficial waters in many developing countries.
In order to optimize its performance, the design of the AATF can be improved to enhance biogas transfer to the liquid phase and also to avoid the accumulation of suspended solids inside the unit.
Conclusions
The novel reactor configuration (aerobic-anoxic trickling filter - AATF) presented promising results for its application in the post-treatment of effluents from anaerobic reactors treating domestic sewage, especially for the removal of dissolved organic matter and nitrogen. However, some changes in the design of the AATF are recommended to avoid biomass accumulation in the interface between the two compartments and also to improve the biogas transfer to the liquid phase and, consequently, to enhance denitrification. The support media was considered adequate for application in AAFT due to its durability and high percentage of void spaces, besides offering favorable conditions for biomass attachment and colonization. Nitrification of the anaerobic effluent was effective in the aerobic compartment. For the applied hydraulic load of 5.6 m3 m-2 d-1 and applied organic load of 0.26 kg COD m-3 d-1 ammonia conversion to nitrate ranged 60 to 74%, and the average ammonia concentration effluent was below 13 mg L-1. A significant fraction of NO3-N was denitrified in the submerged compartment in the presence of the biogas, thus indicating the potential use of the biogas as electron donor for denitrification in such a unit. As the submerged compartment presented DO concentration of about 1 mg L-1, methane might have been the main biogas constituent used as electron donor. Despite the presence of DO, the denitrification was satisfactorily performed and nitrate concentration in the final effluent was under 10 mg L-1.
Referencias
1. F. Qejen, I. Gonenf I. "Criteria for nitrification and denitrification of high-strength wastes in two up flow submerged filters". Water Environ. Res. Vol. 67. 1995. pp. 32-142. [ Links ]
2. M. Strous, E. Van Gerven, P. Zheng, G. Kuenen, M. Jetten. "Ammonium removal from concentrated waste streams with the anaerobic ammonium oxidation (ANAMMOX) process in different reactor configurations". Water Res. Vol. 31. 1997. pp. 1955-1962. [ Links ]
3. M. C. M. Van Loosdrecht, M. S. Jetten. "Microbiological conversions in nitrogen removal". Water Sci. Technol. Vol. 38. 1998. pp. 1-7. [ Links ]
4. J. Bosander, A. Westlund. "Operation of full-scale fluidized bed for denitrification". Water Sci. Technol. Vol. 41. 2000. pp. 115-121. [ Links ]
5. O. Lahav, E. Artzi, S. Tarre, M. Green. "Ammonium removal using a novel unsaturated flow biological filter with passive aeration". Water Res. Vol. 35. 2001. pp. 397-404. [ Links ]
6. K. Than, P. Ajit. "Novel microbial nitrogen removal process". Biotechnol. Adv. Vol. 22. 2004. pp. 519-532. [ Links ]
7. W. M. Rostron, D. C. Stuckey, A. A. Young. "Nitrification of high strength ammonia wastewater: comparative study of immobilization media". Water Res. Vol. 35. 2001. pp. 1167-1178. [ Links ]
8. Environmental Protection Agency (EPA). Process design for nitrogen control. Ed. Office of technology transfer. Washington, USA. 1975. pp. 1-64. [ Links ]
9. H. A. Gullicks, J. Cleasby. "Design of trickling filter nitrification towers". J. Water Pollut. Control Fed. Vol. 58. 1986. pp. 60-67. [ Links ]
10. M. Boller, W. Gujer. "Nitrification in tertiary trickling filters followed by deep bed filters". Water Res. Vol. 20. 1986. pp. 1363-1373. [ Links ]
11. D. Parker, M. Lutz, R. Dahl, S. Bernkopf. "Enhancing reaction rates in nitrifying trickling filters through biofilm control". J. Water Pollut. Control Fed. Vol. 61. 1989. pp. 618-630. [ Links ]
12. W. Okey, O. E. Albertson. "Diffusion's role in regulating rate and masking temperature effects in fixed film nitrification". J. Water Pollut. Control Fed. Vol. 61. 1989. pp. 500-509. [ Links ]
13. E. A. Evans, T. Ellis, H. Gullicks, J. Ringelestein. "Trickling filter nitrification performance characteristics and potential of a full-scale municipal wastewater treatment facility". J. Environ Eng-ASCE. Vol. 130. 2004. pp. 1280-1288. [ Links ]
14. T. C. Zhang, Y. Fu, P. Bishop. "Competition for substrate and space in biofilms". Water Environ. Res. Vol. 67. 1995. pp. 992-1003. [ Links ]
15. D. Parker, T. Richards. "Nitrification in trickling filters". J. Water Pollut. Control Fed. Vol. 58. 1986. pp. 896-902. [ Links ]
16. M. Boller, W. Gujer, M. Tschui. "Parameters affecting nitrifying biofilm reactors". Water Sci. Technol. Vol. 29. 1994. pp. 1-11. [ Links ]
17. Metcalf and Eddy. Wastewater engineering: treatment and reuse. 4th ed. Ed. McGraw-Hill. Inc. New York, USA. 2003. pp. 888-930 [ Links ]
18. R. J. Stenquist, D. Parker, J. J. Dosh. "Carbon oxidation- nitrification in synthetic media filters". J. Water Pollut. Control Fed. Vol. 46. 1974. pp. 2327-2339. [ Links ]
19. G. F. Mehlhart. "Upgrading of existing trickling filter plants for denitrification". Water Sci. Technol. Vol. 30. 1994. pp. 173-179. [ Links ]
20. B. Dorias, P. Baumann. "Denitrification in trickling filter". Water Sci. Technol. Vol. 30. 1994. pp. 181-184. [ Links ]
21. S. Biesterfeld, G. Farmer, L. Figueroa, D. Parker, P. Russell. "Quantification of denitrification potential in carbonaceous trickling filters". Water Res. Vol. 37. 2003. pp. 4011-4017. [ Links ]
22. P. Pearce. "Trickling filter for upgrading low technology wastewater plants for nitrogen removal". Water Sci. Technol. Vol. 49. 2004. pp. 47-53. [ Links ]
23. R. F. Gonjalves, C. Chernicharo, C. De Andrade Neto, P. Sobrinho, M. Kato, R. Da Costa, M. Aisse, M. Zaiat. "Pós-tratamento de efluentes de reatores anaeróbios por reatores com biofilme". Pós-tratamento de efluentes de reatores anaeróbio. Projeto PROSAB. Ed. Belo Horizonte. 2001. pp. 171-199. [ Links ]
24. F. Thalasso, A. Vallecillo, P. García-Encina, F. Fdz- Polanco. "The use of methane as a sole carbon source for wastewater denitrification". Water Res. Vol. 31. 1997. pp. 55-60. [ Links ]
25. J. P. Rajapakse, J. E. Scutt. "Denitrification with natural gas and various new growth media". Water Res. Vol. 33. 1999. pp. 3723-3734. [ Links ]
26. C. Costa, C. Dijkema, M. Friedrich, P. García-Encina, F. Fernandez-Polanco, A. Stams. "Denitrification with methane as electron donor in oxygen-limited bioreactors". App. Microbiol. Biot. Vol. 53. 2000. pp. 754-762. [ Links ]
27. M. I. M. Soares. "Denitrification of groundwater with elemental sulfur". Water Res. Vol. 36. 2002. pp. 1392-1395. [ Links ]
28. S. Islas-Lima, F. Thalasso, J. Gomez-Hernandez. "Evidence of anoxic methane oxidation coupled to denitrification". Water Res. Vol. 38. 2004. pp. 13-16. [ Links ]
29. American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater. 20th ed. Ed. American Public Health Association. Washington, DC. 1998. pp. 1-46 [ Links ]
30. M. Guynot, A. Toribio, M. Quevedo, L. Muxí. "Microflora of dissimilative nitrate reduction in a denitrifying reactor". Appl. Microbiol. Biot. Vol. 50. 1998. pp. 396-400. [ Links ]
31. R. Del Pozo, V. Diez. "Integrated anaerobic-aerobic fixed-film reactor for slaughterhouse wastewater treatment". Water Res. Vol. 39. 2005. pp. 1114-1122. [ Links ]
32. E. Houbron, M. Torrijos, B. Capdeville. "An alternative use of biogas applied in water denitrification". Water Sci. Technol. Vol. 40. 1999. pp. 115-122. [ Links ]
(Recibido el 4 de junio de 2010. Aceptado el 20 de mayo 2011)
*Autor de correspondencia: teléfono: 311 366 26 46, fax: + 54 + 2 + 331 21 75, correo electrónico: jenny.rodriguez@correounivalle.edu.co (J. Rodríguez)