Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230On-line version ISSN 2422-2844
Rev.fac.ing.univ. Antioquia no.60 Medellín Oct./Dec. 2011
Virtual laboratory for simulation and learning of cardiovascular system function in BME studies
Laboratorio virtual para la simulación y el aprendizaje del sistema cardiovascular en estudios de ingeniería biomédica
Alher Mauricio Hernández Valdivieso1*, María Bernarda Salazar Sánchez1, David Alexander Urrego Higuita1, Ramon Costa Castelló2, Miguel Ángel Mañanas Villanueva3
1Grupo de Investigación en Bioelectrónica e Ingeniería Clínica, Programa de Bioingeniería. Universidad de Antioquia. Calle 67 N.° 53-108 Oficina 19-419. Medellín, Colombia.
2Instituto de Organización y Control de Sistemas Industriales, Departamento de Ingeniería de Sistemas, Automática e Informática Industrial, Universitat Politècnica de Catalunya. C.P. 08028. Barcelona, España.
3Centro de Investigación en Ingeniería Biomédica, Departamento de Ingeniería de Sistemas, Automática e Informática Industrial, Universitat Politècnica de Catalunya, C.P. 08028, Barcelona, España.
Abstract
The application of engineering system analysis is a very important field in biomedical engineering (BME) studies: modeling, simulation and control of the most important physiological systems. A virtual laboratory for the analysis and the study of human circulatory system is presented in this paper. This laboratory is based on the compilation of several mathematical models described in the literature. In addition, some model parameters have been tuned by means of experimental data under caffeine stimulus. The computational tool has been built using MATLAB/SIMULINK and EJS, so it combines good computation capabilities with interactivity. The virtual laboratory has been designed in order to understand the operation of the circulatory system under normal conditions, and to predict circulatory variables at different types and levels of stimuli and conditions.
Keywords: Biomedical engineering, interactivity, circulatory system, EJS.
Resumen
La aplicación de la ingeniería al análisis de sistemas es un campo muy importante en los estudios ingeniería Biomédica (BME, por sus siglas en inglés): modelado, simulación y control de los sistemas fisiológicos más importantes. En este documento se presenta un laboratorio virtual para el análisis y el estudio del sistema circulatorio humano. Este laboratorio se basa en la compilación de varios modelos matemáticos descritos en la literatura. Además, algunos parámetros del modelo han sido mejorados por medio de datos experimentales del estímulo de cafeína. Esta herramienta computacional se ha construido utilizando MATLAB / Simulink y EJS, por lo que combina buena capacidad de cálculo con interactividad. El laboratorio virtual ha sido diseñado con el fin de comprender el funcionamiento del sistema circulatorio en condiciones normales, y para predecir variables circulatorias en diferentes tipos y niveles de estímulos y condiciones.
Palabras clave :Ingeniería biomédica, interactividad, sistema circulatorio, EJS.
Introduction
Biomedical Engineering (BME) is different to other engineering areas in the sense of obtaining results from experimental procedures and reproducing real physiological situations. It is very difficult and expensive to interact with the human being body (collaboration of volunteers or patients to be analyzed, instrumentation with specials safety conditions, ethic and legal requirements for the protocols, etc) and even dangerous in certain situations. The use of virtual labs is proposed in order to overcome this drawback and allow students and researchers to interact with the human body. These labs can be built in a complete interactive way, so the students can qualitatively understand the behavior behind the complex models used to represent the human body [1].
The field of BME includes many areas; one of them is the application of engineering principles (modeling, simulation, and control) to biologic problems [2]. In this work the computer modeling of a physiologic system in order to understand its operation under normal situations and to predict physiological variables at different levels of stimuli and conditions is addressed.
The cardiovascular system is composed mainly of the blood, heart, and blood vessels. Blood circulates through out the body to provide individual cells with oxygen and nutrients and helps dispose of metabolic wastes. Arterial pressure control is carried out by a sophisticated non-linear multi-input, multifeedback system termed the baroreflex [3]. The Autonomic Nervous System (ANS) interacts with the cardiovascular system. This interaction and stimuli from the former is critical to understand the function of the later and can provide significant prognostic information. The ANS is predominantly an efferent system transmitting impulses from the Central Nervous System (CNS) to peripheral organs. Its effects include control of Heart Rate (HR), heart contraction, constriction and dilatation of blood vessels, contraction and relaxation of smooth muscle in various organs, and glandular secretions [4].
The virtual laboratory presented in this paper is a useful tool for understanding the cardiovascular system operation, for identifying the stimuli from the ANS and for predicting different situations in a visually attractive and interactive way for the student and researcher on BME.
Methodology
Cardiovascular system model
Empirical and functional models have been proposed in the literature to describe various aspects of the cardiovascular system. Some of them also include the respiratory system interaction through an autonomic neural controller [5]. The cardiovascular system is structured in different intermediate processes (see figure 1). Components in the system include a nonlinear carotid sinus compartment stimulated by the Arterial Blood Pressure (ABP), a sinoatrial node component that regulates the HR with both parasympathetic and sympathetic controls, a peripheral resistance component controlled by the α-sympathetic activity, a hemodynamic submodel based on a simple two-element Windkessel model, and a component that controls changes in stroke volume via changes in the venous return and cardiac contractility [3].
The central autonomic control determines the total α-sympathetic, ftas, β-sympathetic, ftbs, and parasympathetic, ftp, influences on HR and peripheral resistance from the baroreflexes, chemoreflexes and lung stretch receptors reflexes. Autonomic control afferent signals, ftas, ftbs and ftp, allow simulation of different stimuli related with the sympathetic and parasympathetic systems [7].
Sino Auricular Node: Ursino [3] describes the Sino Auricular Node (SA) as a subsystem that translates changes in β-sympathetic and parasympathetic efferent activity into changes in Heart Period, HP (reciprocal of instantaneous HR) which is obtained by assuming a linear interaction between the sympathetic and parasympathetic responses (Equation 1).
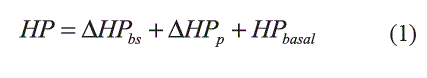
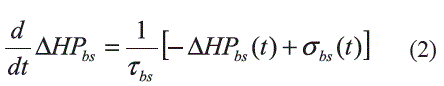
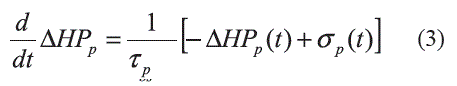
Total Peripheral Resistance (TPR): The α-sympathetic nerves control the peripheral vascular activities. During hypotension/ hypertension, vasoconstriction/vasodilatation occurs to prevent further decreasing/increasing in the blood pressure. This subsystem is modeled using a first-order dynamic system (Equation 4 and Equation 5) as in the case of the (3-sympathetic component:
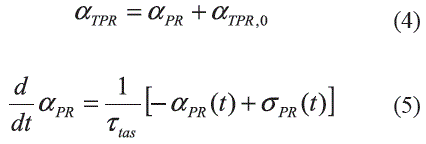
Stroke Volume (SV) and Circulation: The stroke volume and the HP determine the cardiac output, CO (see figura 1). Inputs for this subsystem are the HP, the scaling factor for the TPR, αTPR and the pleural pressure, Ppl. An intermediate variable is the ABP and the resulting output variable in CO. This join with the vasculature modulate ABP. The α-sympathetic nerves control resistance and compliance in the circulation. In the model proposed by Cavalcanti and Belardinelli [6], the circulation subsystem used a two-element Windkessel model where the inputs were the CO and scaling factor for the TPR, αTPR such that the model determines the ABP by means of the following expression (Equation 6):
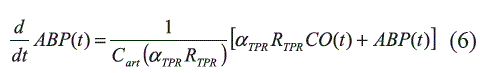
The SV is determined by the venous return, Vn, the HP and the heart contractility, Cn (Equation 7) [8]:

Autonomic nervous system stimuli
Almost every process that affects the autonomic control system also affects the cardiovascular system through the vagal and sympathetic activity. The ANS plays an important role in the regulation of cardiac adaptation during dynamic exercise and during the exposition to certain substances in humans and conscious animals. Five stimuli can be simulated in the virtual laboratory.
Exercise. HR and SV increase during rhythmic or dynamic exercise, which produces an increase in CO. Recent works stated that cardiac sympathetic nerve discharge, ftbs, increases during exercise in proportion to the running speed, and also stated that cardiac sympathetic outflow is stimulated during dynamic exercise although the intensity of exercise is moderate and even low, in concert with cardiac parasympathetic withdrawal [10].
Cholinergic intoxication. It produces increases of parasympathetic system activity, cardiac period (bradycardia) and also hypotension [11]. It is known that this kind of intoxication can be produced by the contact of the respiratory way, digestive or physically with anticholinesterases present in pesticides used in the prevention of plagues that affect the crops or in insecticides. These anticholinesterases inhibit the regulatory function of the acetyl-cholinesterase enzyme, which is responsible of metabolizing the acetylcholine (ACh), what derives in an excess of the mentioned neurotransmitter which implies an over excitation of the parasympathetic receptors, and an increase of the frequency of spikes generated by the above mentioned receptors, ftp.
Caffeine. It is found in coffee, tea, chocolate, and many soft drinks and it keeps us awake by counteracting the effects of inhibitory neurotransmitters. Xanthines such as caffeine and theophylline block adenosine receptors [12]. This effect implies an increase in α-sympathetic and β-sympathetic activity that produces vasoconstriction, higher blood pressure and increased HP and contraction force. This activity is thought to be responsible for the stimulant effects of these agents [13].
Hemorrhage. It is defined as an acute blood volume loss (10% of total or more) and it modifies directly cardiovascular variables like: systemic arterial pressure, CO and total systemic resistance. In addition, it also affects the HR because the system has a baroreceptors feedback that also affects the HR [3].
Scare. Panic disorder is a situation with acute autonomic symptoms such as palpitations, difficulty to breathe, heart pounding, tremulousness and fear of dying because of a heart attack. Thus, the investigation of abnormal cardiovascular function in panic disorder is of specially interest [14]. In healthy people the scare could produce a panic crisis or only a light fear, but in all cases the autonomic system switched to an alert situation characterized by higher α and β sympathetic activity. In addition in patients with panic disorder there is a decrease in cardiac vagal function proportional to the increase in sympathetic function [14].
Identification of caffeine response
In order to simulate each stimulus it is necessary to determine the relationship between the stimulus level and the change of model parameters for the ANS. These parameters were tuned by means of experimental data in the case of caffeine stimulus and were configured according with scientific literature in the remainder ones [10, 11, 14].
Experiment description
Thirteen healthy volunteers (10 women and 3 men, age 23.2 ± 3.5 yr, 164 ± 8 cm, 60.3 ± 9.4 kg), who have not been taking medication, participated in the study. After 2h from their last food intake and 48h of caffeine abstinence they were comfortably installed in a temperature- controlled laboratory. Using a vital signs monitor (CSI model Poet Plus 8100), blood pressure, HR, and oxygen saturation were recorded. In a double-blind, placebo-controlled (descafeined coffee), crossover trial, each subject received a cup of black coffee without sugar (9g diluted in 100ml of water, 153 ± 25 mg/kg) each 20 min, during 1 h.
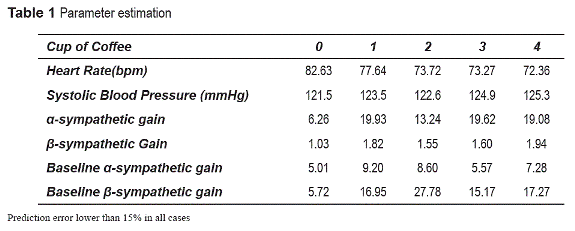
Parameter estimation
Collected mean values of blood pressure and HR during the trial from 0 to 4 cups were used to estimate the change rate of four ANS parameters related with xanthines: gain and baseline of ftas and ftbs via Nelder-Mead Simplex optimization technique [15] with MATLAB. Curves of ftas and ftbs versus coffee cups are included in CardioLab in order to simulate the effect of caffeine in the cardiovascular system. table 1 shows the obtained parameter values in five different stages of the trial and systolic blood pressure and HR variation in experimental data. Prediction error was lower than 15% in all cases. Similar procedures have been implemented in the case of the other stimuli.
Results and discussion
Tool development
Presented software application is based on Easy Java Simulation (EJS) [16], an open source java-based tool that allows creating interactive dynamic simulations.
The circulatory model is quite complex, for that reason the simulation model has been implemented in MATLAB/SIMULINK in order to profit from computation capabilities of this tool. The view and the interactivity have been completely designed and implemented in EJS linked to MATLAB/SIMULINK [17].
Interactive elements
The interactive module of the interface of the Virtual Laboratory is shown in figure 2. Most relevant parameters can be changed by means of sliders and tabs in order to simulate different cardiovascular conditions.
A multisignal scope can be seen in the right side of the interface when the user selects this option (figure 3). Two important types of simulations compose the interactive module:
-Cardiovascular stimuli such as exercise, hemorrhage, scare, caffeine and cholinergic intoxication.
-Sympathetic and Parasympathetic gains: α-sympathetic gain, β-sympathetic gain and parasympathetic gain.
In the first one, user can select the kind of stimulus clicking one of the five tabs available. A representative picture is shown in each tab: a kid with his leg bleeding because of a hemorrhage, a man fumigating without proper protection for cholinergic intoxication, a victim of a terrible scare during a panic attack, a runner when exercise is simulated and a cup of coffee for caffeine as it can be seen in figure 2. User can change the level of stimulus modifying the value of the cardiovascular parameter by means of a slider.
Regarding Sympathetic and Parasympathetic panels, the user can stimulate the cardiovascular system with pharmacological products that produce blockade or hyperactivation of sympathetic or parasympathetic nervous systems. In this panel, user can configure its own stimulation profile. Additionally when the user sets the stimulus level in the five tabs mentioned above the sympathetic and parasympathetic sliders change their value in agreement with the stimulus.
A big animated picture of the heart is shown in the middle of the virtual laboratory and its HP and force of contraction change according to the simulation results. Finally, standard options in virtual laboratories are provided such as to "play", to "pause", to "reset", and to "save" the simulation results ("save-sim"). The last option allows saving the average value of all the variables shown in the "Signal Monitor" in one MATLAB file. It permits further analysis of simulation results or its comparison with experimental data. Furthermore, external windows appear when the user clicks the options "Show Model" (the MATLAB/SIMULINK model is shown) and/or "Signal Monitor".
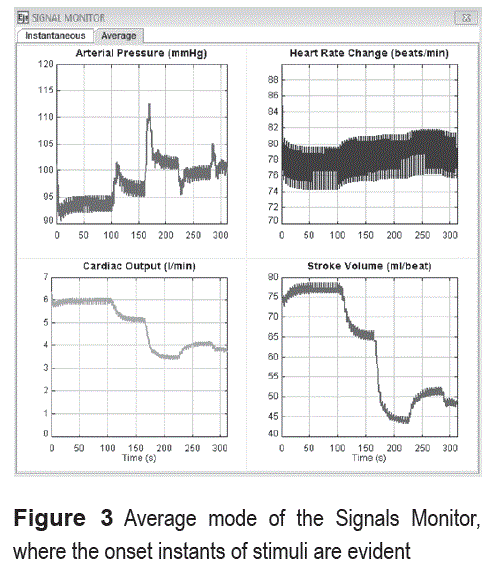
Signal monitor
The user can choose between to different display modes, the instantaneous and the average one. In the former, following variables are shown during the cardiac cycles corresponding to the last 30 seconds: ABP, HR, CO and SV. In the Average mode, the variables are shown from the beginning of the simulation (see figure 3). One illustrative example is used in order to show the capabilities of the tool.
Firstly, a cup of coffee is drunk by the subject (9g diluted in 100ml of water) at 100 seconds. Average signals can be seen in figure 3, where changes in all variables occur in that moment. The ABP increases due to the increased α and β-Sympathetic gains, which develop vasoconstriction as a result of the stimulant effect of xanthines. The HR decreases (HP increases) thanks to the compensation generated by barorreceptors. The cardiac output (CO) increases because of the augmented heart contractility. Sometime after the coffee ingestion, blood pressure and cardiac output remain in a lightly higher value (see figure 3).
Subsequent stimuli applied at intervals of 60 seconds (2, 3 and 4 cups of coffee) increase the effect over ABP and HR.
Conclusions
In this paper a virtual laboratory designed to analyze the human circulatory control system has been introduced. This laboratory is completely graphic and interactive, so it can be used to illustrate the behavior of human circulatory control system under certain circumstances and the influence of relevant parameters in the system. This virtual lab allows obtaining sensations and experiences that would be very difficult otherwise because of the difficulties in performing experimental human studies. The use of virtual laboratories and interactivity in BME has proved to be an efficient way to shortcut the learning process and improve the students capabilities. The tool has been built combining MATLAB/SIMULINK and EJS. While the former allows implementing complex models in straightforward manner, the latter allows designing attractive views and introducing interactivity easily. This combination is quite suitable for virtual laboratory development.
Acknowledgements
This work was partially supported by CODI - University of Antioquia - Colombia (MC09-1- 07), the Spanish government (DPI2010-15110, TEC2008-02754) and International Cooperation Protect (CCD-U014).
References
1. S. Dormido, S. Dormido Canto, R. Dormido, J. Sánchez, N. Duro. "The role of interactivity in control learning". International Journal of Engineering Education. Vol. 21. 2005. pp. 1122-1133. [ Links ]
2. J. D. Bronzino. The Biomedical Engineering Handbook. 2a ed. Ed. CRC Press. Inc. Boca Raton. USA. 2000. pp. 158.1-158.5. [ Links ]
3. M. Ursino. "Interaction between carotid baroregulation and the pulsating heart: a mathematical model". Am. J. Physiol. Vol. 44. 1998. pp. 1733-1747. [ Links ]
4. J. V. Freeman, F. E. Dewey, D. M. Hadley, J. Myers, V. F. Froelicher. "Autonomic nervous system interaction with the cardiovascular system during exercise". Progress in Cardiovascular Diseases. Vol. 48. 2006. pp. 342-362. [ Links ]
5. O. V. Ivanova, M. C. K. Khoo. "Simulation of spontaneous cardiovascular variability using PNEUMA". 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. San Francisco. Vol. 2. 2004. pp. 3901-3904. [ Links ]
6. S. Cavalcanti, E. Belardinelli. "Modeling of cardiovascular variability using a differential delay equation". IEEE TransBiomed Eng. Vol. 43. 1996. pp. 982-989. [ Links ]
7. M. Ursino, E. Magosso. "Role of short-term cardiovascular regulation in heart period variability: a modeling study". Am J Physiol Heart Circ Physiol. Vol. 284. 2003. pp. 1479-1493. [ Links ]
8. K. H. Wesseling, J. J. Settels, W. Wieling, G. A. Van Montfrans and J. M. Karemaker. "The baromodulation hypothesis, baroreflex resetting and one-over-f blood pressure spectra". Computer Analysis of Cardiovascular Signals, chapter 8. M. Di Rienzo, G. Mancia, G. Parati, A. Pedotti and A. Zanchetti (editors). Ed. Burke. VA: IOS Press. Amsterdam. 1996. pp. 105-118. [ Links ]
9. B. J. TenVoorde, Th. J. C. Faes, T. W. J. Janssen, G. J. Scheffer, O. Rompelman. "Respiratory modulation of blood pressure and heart rate studies with a computer model of baroreflex control". Computer analysis of cardiovascular signals, chapter 9. M. Di Rienzo, G. Mancia, G. Parati, A. Pedotti and A. Zanchetti (editors). Ed. Burke. VA: IOS Press. Amsterdam. 1996. pp. 119-134. [ Links ]
10. H. Tsuchimochi, K. Matsukawa, H. Komine, J. Murata. "Direct measurement of cardiac sympathetic efferent nerve activity during dynamic exercise". Am J Physiol Heart Circ Physiol. Vol. 283. 2002. pp. 1896-1906. [ Links ]
11. O. A. Timofeeva, D. Sanders, K. Seemann, L. Yang, D. Hermanson, S. Regenbogen, S. Agoos, A. Kallepalli, A. Rastogi, D. Braddy, C. Wells, C. Perraut, F. J. Seidler, T. A. Slotkin, E. D. Levin. "Persistent Behavioral Alterations in Rats Neonatally Exposed to Low Doses of the Organophosphate Pesticide, Parathion". Brain Res Bull. Vol. 77. 2008. pp. 404-411. [ Links ]
12. J. C. Shryock, L. Belardinelli. "Adenosine and adenosine receptors in the cardiovascular system: Biochemistry, physiology, and pharmacology". The American Journal of Cardiology. Vol. 79. 1997. pp. 2-10. [ Links ]
13. B. B. Fredholm. "Adenosine, adenosine receptors and the actions of caffeine". Pharmacol. Toxicol. Vol. 76. 1995. pp. 93-101. [ Links ]
14. V. K. Yeragani, M. Tancer, T. Uhde. "Heart rate and QT interval variability: abnormal alpha-2 adrenergic function in patients with panic disorder". Psychiatry Research. Vol. 121. 2003. pp. 185-196. [ Links ]
15. Y. Huang "An improved simplex method for function minimization." IEEE Int. Conf. Systems, Man and Cybernetics Beijing. Vol. 3. 1996. pp. 1702-1705. [ Links ]
16. F. Esquembre. Creación de simulaciones interactivas en Java. Ed. Pearson Education -Prentice Hall. Madrid. 2004. pp. 19 -352. [ Links ]
17. J. Sánchez, F. Esquembre, C. Martín, S. Dormido, R. Dormido, S. Dormido-Canto, R. Pastor. "Easy java simulations: an open-source tool to develop interactive virtual laboratories using matlab/simulink". International Journal of Engineering Education. Vol. 21. 2005. pp. 798-813. [ Links ]
(Recibido el 1 de octubre de 2010. Aceptado el 14 de abril de 2011)
*Autor de correspondencia: teléfono:+ 57 + 4 + 219 85 88, correo electrónico: mauricio.hernandez@udea.edu.co (A. M. Hernández)