Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Facultad de Ingeniería Universidad de Antioquia
versão impressa ISSN 0120-6230versão On-line ISSN 2422-2844
Rev.fac.ing.univ. Antioquia n.62 Medellín jan./mar. 2012
ARTÍCULO ORIGINAL
Yeast immobilization in lignocellulosic wastes for ethanol production in packed bed bioreactor
Inmovilización de levaduras en residuos lignocelulósicos para la producción de etanol en biorreactor de lecho empacado
Lina María Agudelo Escobar1*, Uriel Salazar Álvarez1, Mariana Peñuela2
1Grupo de Biotransformation. Escuela de Microbiología. Universidad de Antioquia. Calle 67 No.53-108. Bloque 5 laboratorio 226. Medellín, Colombia.
2Grupo de Biotecnología. Sede de Investigación Universitaria (SIU). Universidad de Antioquia. Calle 62 N° 52 - 59 Torre 1 laboratorio 210. Medellín, Colombia.
*Autor de correspondencia: teléfono: + 57 + 4 +219 84 83, fax: + 57 + 4 + 219 10 07, correo electrónico: agudelo.linamaria@gmail.com (L. Agudelo)
(Recibido el 5 de abril de 2011. Aceptado el 24 de febrero de 2012)
Abstract
This study is focused on the development of an immobilization process of yeast cells in waste lignocellulosic materials and their evaluation in the ethanol production by using packed bed bioreactors. We evaluated four different waste lignocellulosic materials: wood shavings, cane bagasse, corn cobs and corn leaves. The characterization made it possible to establish the microscopic structural differences between the four materials, as well as the differences in composition of lignin, cellulose, hemicelluloses and ash. A protocol for packaging materials and a quantification methodology of immobilized biomass was developed. An experimental design was conducted to determine the effects of the size of lignocellulosic materials on cell immobilization and to establish the effects of the flow rate in the immobilized cells when the fermentation is performed in packed bed bioreactors. Under the established experimental conditions we determined the size and flow rate that provided the better operational stability for the fermentation in packed bed bioreactor.
The result of the study showed that the material in which the biggest amount of cells was immobilized was the sugar cane bagasse, and we obtained a value of 0.04657 dry immobilized biomass gX/gS (grams of dry biomass per grams of lignocellulosic material). This amount of immobilized biomass is significant compared to the values reported by other authors. As a result of the experimental design of the influence of flow and size of the carrier on the immobilization, it was established that there is no significant statistical difference in the range of the values used in the experiment (size of 3.4; 6.7 and 10 mm and flow rate of 0.36; 1.6 and 3.33 ml/s), so the best conditions for cell immobilization could be established taking into account the operational conditions and system stability. The size of the carrier selected was 3.4 mm and the flow rate of 0.36 ml/s. In the fermentations carried out in packed bed bioreactors with the biocatalyst (carrier + yeast), it was possible to obtain an increase of the amount of immobilized cells up to a value of 0.1444 ± 0.0061 grams of biomass after 12 hours of fermentation. Ethanol production using these biocatalysts reached a value of 20 g/L in 12 hours fermentation batch, equivalent to a volumetric productivity of 1.67 g/Lh.
The results have established the implementation potential of ethanol production by continuous fermentation with immobilized cells in waste lignocellulosic materials.
Keywords: Immobilization yeasts cells, bioethanol, biofuels, lignocellulosic carriers, continuous fermentation
Resumen
El presente estudio se ha orientado hacia el desarrollo de un proceso de inmovilización de células de levaduras en residuos de materiales lignocelulosicos y a su evaluación en la producción de etanol mediante el uso de biorreactores de lecho empacado. Se evaluaron cuatro diferentes residuos de materiales lignocelulosicos: viruta de madera, bagazo de caña, tusa y capacho de maíz. La caracterización realizada permitió establecer las diferencias estructurales microscópicas que presentan los cuatro materiales, al igual que las diferencias en composición de lignina, celulosa, hemicelulosa y cenizas. Se desarrolló un protocolo para el acondicionamiento de los materiales y una metodología de cuantificación de biomasa inmovilizada. Se realizó un diseño experimental para determinar el efecto del tamaño de los materiales lignocelulósicos en la inmovilización celular y para establecer el efecto del caudal empleado en el proceso de inmovilización en los biorreactores de lecho empacado. Bajo las condiciones experimentales establecidas se determinó el tamaño y caudal que mejor estabilidad operacional proporcionaron a la fermentación en el biorreactor de lecho empacado.
Como resultado del estudio se obtuvo que el material en el que se inmovilizó mayor cantidad de células de levadura fue el bagazo de caña, se obtuvo un valor de biomasa seca inmovilizada de 0,04657 gX/gS (gramos de biomasa por gramos de material lignocelulósico empleado). Esta cantidad de biomasa inmovilizada es significativa comparada con los valores reportados por otros autores. Como resultado del diseño experimental de la influencia del caudal y el tamaño del soporte en la inmovilización se pudo establecer que no existe diferencia estadística significativa en el rango de valores empleados en la experimentación (tamaño de 3,4, 6,7 y 10 mm y caudal de 0,36, 1,6 y 3,33 ml/s), así que las mejores condiciones para la inmovilización celular pudieron ser establecidas teniendo en cuenta las condiciones operacionales y estabilidad del sistema, el tamaño de soporte empleado fue de 3,4 mm y el caudal de 0,36 ml/s. En las fermentaciones realizadas en los biorreactores de lecho empacado con el biocatalizador (soporte + levaduras), se logró obtener un aumento en la cantidad de células inmovilizadas hasta un valor de 0,1444 ± 0,0061 gramos de biomasa inmovilizada después de 12 horas de fermentación. La producción de etanol empleando estos biocatalizadores alcanzó un valor de 20 g/L en 12 horas de fermentación por lote, es decir, una productividad volumétrica de 1,67 g/Lh.
Los resultados obtenidos han permitido establecer el potencial de la implementación de la producción de etanol mediante fermentaciones en continuo con células inmovilizadas en residuos de materiales lignocelulosicos.
Palabras clave: Inmovilización de células de levadura, bioetanol, biocombustibles, materiales lignocelulósicos, fermentación en continuo
Introduction
High oil prices and decrease of world stock, in addition to the concerns about the continuous deterioration of the environment, have given a strong push to the search for alternative energy sources in recent years, particularly the development of liquid biofuels to replace oil and its derivatives, and reduce the negative impact on the atmosphere caused by burning fossil fuels. In the last two decades there has been an increase of research focused on developing new technologies for ethanol production from renewable sources, and the efforts have focused towards the development of much more efficient production processes [1- 3].
Most industrial processes for ethanol production use batch systems, which are being implemented in Colombia in new production plants [4]. Some of the disadvantages of these processes are: the high capacity required for fermentation, the variability of the composition of the medium during the process, the existence of non-productive stages (e.g. stages of loading and downloading) and the need to continuously centrifuge the yeast for its reuse.
As an alternative, continuous processes are brought up, which can reduce production costs, improve process efficiency and increase ethanol yield. Continuous processes that use immobilized cells show great advantages over those using free cells, since they facilitate the separation of the product, allow re-use of the biocatalyst, they have a high volumetric productivity, and facilitate the control of process variables as well as reducing the risk of contamination [5].
However, fermentations with immobilized cells also have some disadvantages, such as the difficulty to predict changes in cell growth, physiology and metabolic activity, and the presence of mass transfer limitation by diffusion. Other authors have reported improvements [6, 7]. There are also demanding requirements for materials used as carriers, since chemical, physical and biological stability has to be ensured, and the carriers and the technique must be low cost and easy to implement on an industrial scale.
Among the most widely used immobilization matrices are hydrophilic polymer gels such as alginate, carrageenate, agarose, etc. where cells are immobilized by entrapment in the gel. However, this method of immobilization is impractical on an industrial scale because of the cost of raw materials and the relative complexity in the preparation of biocatalysts with respect to the operational lifetime in the process [8- 10]. Other methods of immobilization in relation to the union of the cells to the carrier are: adsorption, covalent bonding, crosslinking and entrapment between membranes [11]. In recent years, raw materials derived from agro-industrial waste and their application as promising carriers for the immobilization of cells have been evaluated. For instance, the sugar cane bagasse [12], delignified cellulosic materials [13], orange peel [14], spent grains [15, 16], wood chips [17], among others. On these carriers the cells are immobilized by adsorption, a simple and economically favorable technique. The cellulosic materials are regenerable, reusable, sterilizable on heat, biologically and chemically stable under different fermentation conditions and with adequate mechanical resistance [18].
The main mechanisms that occur during immobilization by adsorption are: i) adsorption in the cavities of the carrier by mechanical retention and colony formation in macropores; ii) aggregate formation, by binding of cells, flocculation and formation of chains, iii) adherence to the solid surface by covalent, ionic, hydrogen bonding, hydrophobic or biochemical interactions between cells and the matrix. Most of these mechanisms are spontaneously generated during contact between cells and carrier, so adsorption is one of the simplest mechanisms to perform, both in a laboratory and industrial level.
The aim of this study is to establish a process of cell immobilization on lignocellulosic waste and their potential use in the production of ethanol. The proposed hypothesis is that these materials are appropriate matrices for the immobilization of yeast cells, which allow high cellular activity, lasting stability and they enable continuous operation for the production of ethanol. The lignocellulosic wastes used in this research are obtained from agro-industries and they are abundant in our country.
Methodology
The organism and culture media
In this work we used the commercial yeast Saccharomyces cerevisiae ethanol RED®. The strain was kept lyophilized and stored at 4°C.
The medium for inoculum and fermentation is composed of: glucose 100 g/L, (NH4) 2SO4, 5 g/L, yeast extract, 3g /L, KH2PO4, 2 g/L and MgSO4.7H2O, 1 g/L. The pH was adjusted to 5.0.
Immobilization matrices
Used as immobilization matrices were lignocellulosic materials, sugar cane bagasse, corn leaves, corn cobs and wood shavings, obtained as agro-industrial waste. These materials were subject to a conditioning process that involved the implementation of a protocol for cleaning, drying and size reduction. The average sizes of the pieces obtained were 3.4; 6.7 and 10 mm.
Immobilization of yeast
(or immobilization baseline), used as matrices for immobilization of Saccharomyces cerevisiae were, the pieces of lignocellulosic material obtained previously, which were packed into bioreactors to occupy 70% of the reactor volume. The immobilization was carried out by continuous recirculation of a cell suspension with 15 g/L of biomass for a period of 12 hours. The process was carried out at 30°C in 150 ml bioreactors.
Biomass quantification Protocol (dry weight modified technique)
After completion ofthe 12 hours of immobilization baseline, the biomass quantification process developed was as follows: the liquid was decanted in the bioreactor; there were four successive washes with isotonic solution to eliminate cells that were deposited on the carrier and the bioreactor, but not immobilized. Then the biocatalyst (cells + carrier) was dried at 105°C for 12 hours. Drying time elapsed, biocatalysts were left idle until they reached room temperature, then 9 samples of each one were taken, with an approximate weight of 0.3g and they were distributed in Falcon tubes. Series of three tubes were filled with 50 ml of water, isotonic solution and NaOH 0.1% (w/v). De-inmobilization was performed by mechanical agitation at 150 rpm for 24 hours at room temperature. After this process all carriers were washed with isotonic solution and left drying for 12 hours at 105°C. After drying, they were weighed again. The same process was implemented with cell-free material to be used as blank. The immobilized biomass was obtained from the dry weight difference of the carrier before and after the de-immobilization process, and the value was corrected using the blank. For the development of the protocol three solutions were evaluated: water, NaOH 0.1% (w/v) and isotonic solution. Each treatment was performed in triplicate.
Study of the effect of size and flow rate for each material
To determine the effect on immobilization of flow rate and the carrier size, we performed a factorial experimental design with 2 factors and 2 levels, and a central point with three replicates. The results of this experiment were analyzed using the statistical analysis program Design Expert.
Immobilization under fermentative conditions in 250 mL bioreactor
We used a glass column of 250 ml capacity, fitted jacket for temperature control and two peristaltic pumps for feeding fresh culture medium and circulation of water through the jacket. The equipment was sterilized and loaded with pieces of biocatalyst up to 70% capacity. The baseline immobilization was performed by following the yeast immobilization protocol. Subsequently, the bioreactor was filled with fresh culture medium and allowed to operate with recirculation for 12 hours [19]. The response variables evaluated in this study were the reduced sugars consumption, and ethanol production.
Analytical methods
Microbial biomass was determined by the dry weight modified protocol developed in this research. The reduced sugars in the culture medium were determined using the technique of DNS [20]. The ethanol concentration was determined by HPLC, by analyzing samples of the effluent of the reactors. We used a column Supelco gel C610H with the following conditions: 20 µL of injection volume, phosphoric acid 0.1 % as mobile phase, flow 0.5 ml/min., and an oven temperature of 30°C. The method of detection was rate refraction, and the retention time was 26.3 min. The Scanning Electron Microscopy technique (SEM) was used to observe the differences in the structures of materials and cell immobilization. Determination of lignin, cellulose, hemicellulose and ash contents was carried out by the detergent method of Van Soest [21].
Results and discussion
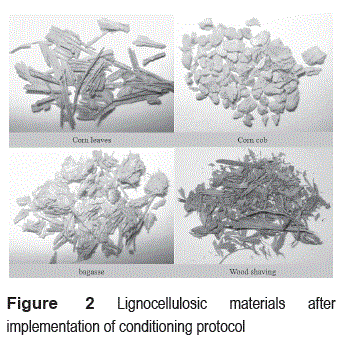
Due to heterogeneity in nature and origin of agro-industrial waste used in the experiment, and to avoid interference in the subsequent process of cell immobilization, it was necessary to establish a cleaning treatment and adapting materials. Figure 1 presents the protocol developed for this treatment, which consisted mainly in successive washes with hot water, dried in an oven at 105°C and size reduction carried out by considering mass transfer criteria for packed bed reactors.
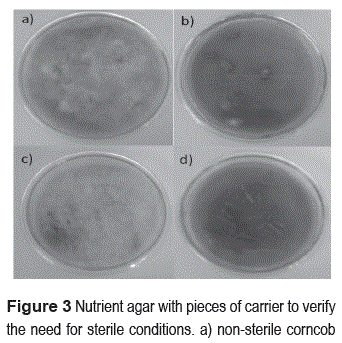
Figure 2 shows the material upon completion of the process of conditioning. Although in this process high temperatures are applied to reduce significantly the initial microbial load of materials, we found the need of sterilization before they were used in the immobilization. Figure 3 shows the growth of microorganisms in petri dishes containing nutrient agar and pieces of non-sterile materials; however, there is no growth in the petri dishes that were placed in sterile lignocellulosic residues.
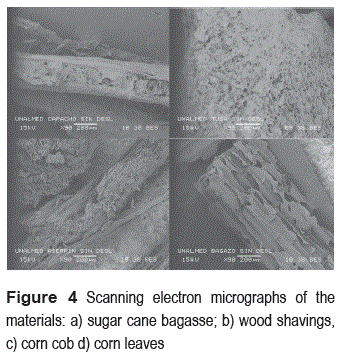
Figure 4 presents the results of the images obtained from scanning electron micrographs (SEM). Differences on the surface of the structures of the four materials can be found, these differences can lead to changes in yeast cell immobilization. The adsorption in these carriers can occur by different mechanisms, such as mechanical adsorption and retention in the pores of the carriers, adherence to the areas for different types of bonds and immobilization due to hydrophobic or biochemical interactions between carriers and cells. These immobilization mechanisms are not only dependent of the surface characteristics of the carriers, they depend on cell surface characteristics, the carrier used and the operational conditions under which the immobilization is carried out (flow, temperature, etc.). To establish which of these mechanisms were involved in immobilization on the lignocellulosic materials used, it is necessary to study each of these characteristics.
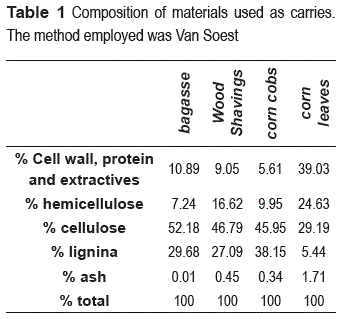
Regarding materials composition, the percentages of lignin, cellulose, hemicellulose and ash that are presented in table 1, there are different proportions in each material; these differences can directly affect yeast immobilization. For example, lignin values of sugar cane bagasse, wood shavings and corncob are high, 29.68; 27.09 and 38.15% respectively, compared to the corn leaves on which only has 5.44 %. Lignin is the substance that gives stability and rigidity to lignocellulosic material, so the low value for corn leaves may cause a structural weakness of material, it could affect the continuous operation for long periods, reducing their potential for use in immobilization processes on an industrial level.
To establish immobilization capacity on each of the materials and determine the effect of size and flow rate in the process, we standardized the biomass quantification methodology on the carriers. We studied various techniques (spectrophotometry, protein quantification and dry weight); the better results were obtained with the dry weight technique, which was modified to allow direct quantification of biomass immobilized on the carrier. The protocol developed was described in the methodology (biomass quantification protocol). By this methodology it was possible the direct determination of immobilized biomass ensuring reproducibility in the quantification.
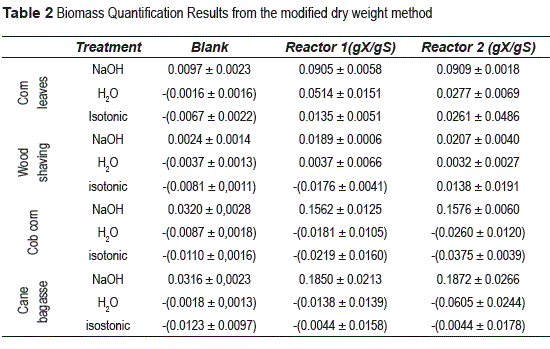
Table 2, presents the results in this standardization, when we used the biomass quantification protocol proposal. In the first column the carriers studied are listed (corn leaves, wood shavings, corn cob and cane bagasse). Treatment column corresponds to the different solutions evaluated (NaOH 0.1%(w/v), water and isotonic solution). Blanks were evaluated using cell-free material. Reactor1 and Reactor 2 columns correspond to experiments performed in reactors 1 and 2 respectively, where (gX/gS) are grams of biomass immobilized per gram of carrier used.
As shown, the values obtained in the two reactors for treatment using water and isotonic solutions are not suitable for immobilized biomass determination. The results are not valid for the proposed protocol, because the negative values represent high quantity of cells on the carrier at the end of the de-immobilization process. In addition, the results have high deviations.
However, the treatments with NaOH 0.1% (w/v), showed results consistent with established protocol and they have excellent reproducibility with very low standard deviations values. Based on these results we chose the biomass quantification protocol (modified dry weight treatment), that uses NaOH 0.1% (w/v), as the best methodology for the quantification of immobilized biomass on the carriers.
The experimental design was developed to determine the appropriate size and flow rate used in alcoholic fermentation in a 250 ml bioreactor. In the experimental design, 15 treatments were evaluated by combining three sizes (3.4; 6.7 and 10 mm) and three flow rates (0.36; 1.6 and 3.3 ml/s) for each material. To determine the size range of the carriers, we considered the bioreactor used and mass transfer criteria for packed bed columns. For flow rate we considered the specifications of peristaltic pumps available in the laboratory and the residence time established previously in the bioreactor used.
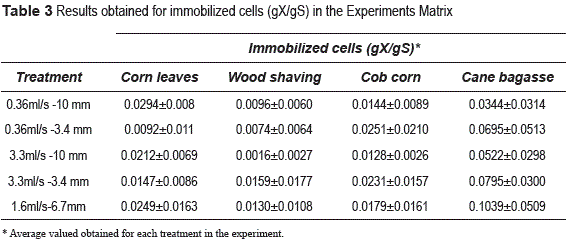
Table 3 presents the consolidated experiments matrix with the results obtained. We can see that the amount of immobilized cells varies depending on the treatment and the material. The high variability in each of the treatments for different materials is indicated by the high deviations values and it is confirmed by the lack of statistical significance.
The experimental design analysis using Design Expert software allowed us to establish that there is no significant statistical difference between treatments. ANOVA P-value of 0.3908; 0.3394; 0.3474 values were obtained for the experimental designs of wood shaving, corn leaves and bagasse, respectively. For the corn cob carrier, we obtained a P-value of 0.0237, which indicates statistical significance; although in this material it was not possible to achieve a high quantity of immobilized cells. Based on these results, the best choice of flow rate and size for cell immobilization can be established considering the best operating conditions of the reactor, because the values of the studied ranges for flow rate and size have no effect on the amount of immobilized biomass in the carriers.
An important result obtained from the experimental design, is about selection of the best material for cell immobilization. As shown in the results of table 3, it is the cane bagasse the material on which the biggest quantity of biomass was immobilized. The treatment corresponding to a flow rate of 1.6 ml/s and a carrier size of 6.7mm obtained a cell immobilization of 0.1039 gX/ gS, which is the largest amount of immobilized cells obtained so far. Based on these results, we chose the sugar cane bagasse as the carrier for immobilization in fermentation conditions.
The batch fermentation elapsed time was 12 hours and it was performed in a bioreactor in triplicate. As a result we obtained 0.1444 ± 0.0061 gX/gS. These values are significant if compared with the data reported by others researchers, who are using different lignocellulosic materials as carriers and obtained 0.02 gX/gS, after 75 hours of continuous processes of immobilization [12].
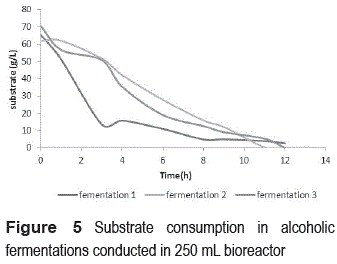
Figure 5 shows the results for substrate consumption of three different fermentations carried out with immobilized cells. The consumption of substrate for fermentation 1 is performed in a shorter time -about 3 hours after the start of the fermentation process the sugars are reduced by 80%. The behavior of fermentations 2 and 3 is different, we observed that sugar consumption is slower and achieves 80% reduction in the substrate concentration after approximately 8 hours of fermentation. An important result in this experimentation was the confirmation of reproducibility of operational conditions of fermentations and stability of packed bed system during operation time.
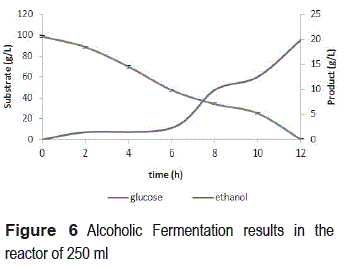
Figure 6 shows the results for the kinetics of substrate consumption and ethanol production in batch fermentation. There is a similar behavior in terms of substrate consumption obtained in the fermentations presented in figure 5. Between 8 and 10 elapsed hours the glucose consumption was 80%. Ethanol production began in the early hours of fermentation, but only provided an exponential accumulation by 6 hours after the process started. At the end of the fermentation it is observed that there is a complete exhaustion of the substrate and production of 20g/L of ethanol.
These results indicate that, despite of the total absence of sugars at the end of fermentation, not all of them were converted to ethanol. The performance of the alcoholic fermentation is approximately 39% of the theoretical value. It is important to mention that continuous research is required, in order to optimize the amount of immobilized biomass on the support and the amount of biocatalyst packed in the reactor, and to obtain the appropriate inoculum size to achieve higher productivity in the fermentation. Anaerobic conditions must be ensured as well, so that the substrate consumption is oriented to the production of ethanol and not to increase biomass quantity during fermentation. In additional experiments, we were able to establish that the carriers absorb some of the sugars in the culture medium, decreasing the amount of sugars available to be processed into alcohol, and therefore decreasing the performance of the fermentation. However, despite this fact, it was found out that the absorption of sugars reaches a saturation point beyond which no absorption occurs and the concentration of sugars in the culture medium is kept constant.
Since the packed bed bioreactor system has great operational stability and the results of fermentation with a volumetric productivity of 1.67 g/Lh and a biomass of 4.1 g/L of immobilized biomass are comparable with those reported in batch fermentations with free cells, which use twice the amount of biomass, the immobilization cell in lignocellulosic materials has a high potential for alcoholic fermentation on industrial scale. However, to achieve better results and be able to implement this system at industrial level, it is necessary to optimize the process to establish the best concentrations of sugars, inoculum size and biocatalyst quantity appropriate for the bioreactor, improving the yields and making the process competitive with the actual existent ethanol production technology.
Conclusions
In this study we developed a protocol for quantification of immobilized biomass on lignocellulosic carriers and we obtained reproducible results. The choice of the carrier sizes and flow rates for immobilization in fermentation conditions within the studied ranges can be made by considering the best operating conditions. There is a great potential of lignocellulosic materials (wood shavings, bagasse, corn leaves and corn cob), for use as carriers in the immobilization on an industrial scale, because the immobilization is easy, agro-industrial waste materials are found in large amounts and the quantity of immobilized biomass obtained is significant compared with the data reported by other authors. This demonstrates that if further investigations are carried out to establish the best times for the immobilization process and the best fermentation conditions, high yields of ethanol production can be reached by using immobilized cells in the carriers studied. Analytic techniques are also required for measuring the physicochemical properties of yeast cells and carriers, to determine the mechanisms of immobilization that occur in the process.
Referencias
1. L. Mojovic, S. Nikolic, M. Rakin, M. Vukasinovic. ''Production of bioethanol from corn meal hydrolyzates''. Fuel. Vol. 85. 2006. pp. 1750-1755. [ Links ]
2. L. Chaparro, M. Cuervo, J. Gómez, M. Toro. Releases to the environment in Colombia. IDEAM web publications. Disponible en: http://www.ideam.gov.co/publica/index4.htm. Consultado el 10 de Julio de 2009. [ Links ]
3. M. Krishnan, F. Taylor, B. Davison, N. Nghiem. ''Economic analysis of fuel ethanol production from corn starch using fluidized-bed bioreactors''. Bioresource Technology. Vol. 75. 2000. pp. 99-105 [ Links ]
4. Ministerio de Minas y Energía. Biocombustibles. Memorias al Congreso Nacional. 2005-2006. pp. 62-66. [ Links ]
5. R. Wendhausen, A. Fregonesi, P. Moran, I. Joekes, J. Rodrigues, E. Tonella, K. Althoff. ''Continuous Fermentation of Sugar Cane Syrup Using Immobilized Yeast cells''. Journal ofBioscience and Bioengineering. Vol. 91. 2001. pp. 48-52. [ Links ]
6. Q. Jiang, S. Yao, L Mei. ''Tolerance of Immobilized Baker's Yeast in Organic Solvents''. Enzyme Microbiology and Technology. Vol. 30. 2002. pp.721-725. [ Links ]
7. J. Hallsworth. ''Ethanol-induced Water Stress in Yeast''. Journal of Fermentation and Bioengineering. Vol. 85. 1998. pp.125-137. [ Links ]
8. D. Serp. From Whole Cell Immobilization: Practical Insight To Tools Applications. PhD Thesis. École Polytechnique Fédérale De Lausanne. Switzerland. 2002. pp. 1-50. [ Links ]
9. L. Kurillová, P. Gemeiner, A. Vikartovská, H. Mikov, M. Rosenberg, M. Ilavsky. ''Calcium pectate gel beads for cell entrapment. 6. Morphology of stabilized and hardened calcium pectate gel beads with Immobilized cells for biotechnology''. Journal of Microencapsulation. Vol. 17. 2000. pp. 279-296. [ Links ]
10. V. Martins dos Santos, E. Leenen, M. Rippoll, C. Sluis, T. Vliet, J. Tramper, R. Wijffels. ''Relevance of rheological properties of gel beads for mechanical Their Stability in bioreactors''. Biotechnology and Bioengineering. Vol. 56. 1997. pp. 517-529. [ Links ]
11. C. Champagne, C. Lacroix, I. Sodini-Gallot. ''Immobilized cell technologies for the dairy industry''. Critical Reviews in Biotechnology. Vol. 14. 1994. pp. 109-134. [ Links ]
12. J. Vasconcelos, C. Lopes, F. França. ''Continuous Ethanol Production Using Immobilized Yeast On Sugar-Cane Stalks''. Brazilian Journal of Chemical Engineering. Vol. 21. 2004. pp. 357-365. [ Links ]
13. Y. Kourkoutas, C. Psarianos, A. Koutinas, M. Kanellaki, I. Banat, R. Marchant. ''Continuous Whey Fermentation Using Kefir Yeast Immobilized on Delignified Cellulosic Material''. Journal of Agricultural of Food Chemistry. Vol. 50. 2002. pp. 2543-2547. [ Links ]
14. S. Plessas, Y. Kourkoutas, C. Psarianos, M. Kanellaki, A. Koutinas. ''Continuous baker's yeast production using orange peel as Promoting support in the bioreactor''. Journal of the Science of Food and Agriculture. Vol. 86. 2005. pp. 407-414. [ Links ]
15. T. Brányik, A. Vicente, J. Machado, J. Teixeira. ''Spent grains - a new support for brewing yeast Immobilization''. Biotechnology Letters. Vol. 23. 2001. pp. 1073-1078. [ Links ]
16. G. Dragone, S. Mussatto, J. Almeda e Silva. ''Influence of temperature on continuous high gravity brewing with yeasts immobilized on spent grains''. European Food Research and Technology. Vol. 228. 2008. pp 257-264. [ Links ]
17. M. Linko, I. Virkajarvi, N. Pohjala, K. Lindborg, J. Kronlof, E. Pajunen. Main Fermentation with Immobilized Yeast-A Breakthrough?. Proceedings of the European Brewing Convention Congress. Oxford (USA). 1997. pp. 385-394. [ Links ]
18. T. Brányik, A. Vicente, P. Dostálek, J. Teixeira. ''Continuous Beer Fermentation Using Immobilized Yeast Cell Bioreactor Systems''. Biotechnology Progress. Vol. 21. 2005. pp. 653-663. [ Links ]
19. G. Vargas, M. Peñuela, M. Echeverri, M. Ortiz, M. Escobar, J. Quintero. ''Alcohol production by fermentation using free and immobilized yeast''. Revista Facultad de Ingeniería Universidad de Antioquia. No. 22. 2001. pp. 91-97. [ Links ]
20. G. Millar. ''Use of DNS for determination of Reducing Sugar''. Analytical Chemistry. Vol. 3. 1959. pp. 426428. [ Links ]
21. P. Van Soest, J. Robertson, B. Lewis. ''Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in Relation to animal nutrition''. Journal of Dairy Science. Vol. 74. 1991. pp. 3583-3597. [ Links ]