Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230On-line version ISSN 2422-2844
Rev.fac.ing.univ. Antioquia no.62 Medellín Jan./Mar. 2012
ARTÍCULO ORIGINAL
Phase diagram of polymer electrolyte: (x)(PEO) - ( 1-x)CF3COOLi
Diagrama de fases del polímero electrolito: (x) (PEO) - (1 - x)CF3COOLi
Miguel Iban Delgado1*, Nori Magali Jurado1, Rubén Antonio Vargas2
1Departamento de Física. Universidad del Tolima. A.A. 546. Ibagué, Colombia.
2Departamento de Física. Universidad del Valle. A.A. 25360. Cali, Colombia.
*Autor de correspondencia: teléfono: +57 + 8 + 266 92 62, correo electrónico: mirosero@ut.edu.co (I. Delgado)
(Recibido el 11 de Marzo de 2011. Aceptado el 28 de febrero de 2012)
Abstract
Solid polymer electrolytes based on poly(ethylene oxide) (PEO) and lithium trifluoroacetate (CF3COOLi) were prepared by a solvent casting method by different concentrations of salt. The membranes resulting of slow evaporation were analyzed by differential scanning calorimetry (DSC) and its phase diagram was drawing for a second thermal scan. The DSC curves on the first thermal cycle show three anomalies at 303, 338 and 393 K, that are slightly shifted with the salt content. For high concentrations, the intensity of the endothermic peak associated with the melting point of PEO (338 K) decreases, that one for the third transition increases with the salt content, the second peak (melting point of PEO) disappear when the concentration reaches the value of X = 0,405 (given in fraction of mass: salt/(salt + polymer)). These results and the change of conductivity demonstrate the formation of a new complex between the PEO and the CF3COOLi.
Keywords: Poly(ethylene oxide), ionic conductivity, polymer electrolytes
Resumen
A partir de poli(oxido de etileno) y trifluoro acetato litio (CF3COOLi), se prepararon electrolitos sólidos poliméricos por el método de solución, para diferentes concentraciones de sal. Las membranas resultantes de la evaporación lenta, se analizaron por calorimetría diferencial de barrido (DSC) y se realizó un diagrama de fases a partir de los segundos calentamientos. Las curvas de DSC para un primer barrido muestran tres anomalías a 303, 308 y 393 K, las que cambian lentamente con el contenido de sal. Para altas concentraciones, la intensidad del pico endotérmico asociado con el punto de fusión del PEO (338K) decrece, mientras que para la tercera anomalía (asociada a la fusión del complejo PEO:sal) la intensidad aumenta. La segunda anomalía desaparece por completo, cuando la concentración de sal alcanza un valor de X = 0,405 (dada en fracción de masa: sal/(sal + PEO)). Estos resultados junto alta conductividad del sistema, muestran la formación de un nuevo complejo entre el PEO y la sal.
Palabras clave: Poli (óxido de etileno), conductividad iónica, polímeros electrolitos
Introduction
Polymer electrolytes have been the focus of intense research efforts due their potential application in rechargeable batteries and electrochemical sensors [1, 2]. Systems of poly (ethylene oxide) PEO with different lithium, sodium and potassium salts have received considerable attention, since they exhibit solid phases with high ionic conductivity at room temperature.
The high conductivity is assigned to the amorphous phase of the polymeric matrix, assisted by the segmental motion of the polymeric backbone [3, 4]. With this in mind, other authors have used the route of increasing the flexibility of the polymer chains, by preparing crosslinked polymer networks in block and comb-like structures [5, 6].
Is well known that PEO forms complex with inorganic salts, where cations are coordinated by several oxygen atoms from helical polymer chain winding around cation [7,8]. Crystalline complexes formed have typically melting temperature higher than PEO (e.g. 452 K for PEO3:LiCF3SO3 and 353 K for PEO3:LiN(CF3SO2)2) [9], In g eneral, amorphous polymer salt complexes exhibit higher conductivity compared with their crystalline counterparts. Flexibility on the chains, which is higher in amorphous phase, is crucial in improving the ionic transport. The spectroscopic studies indicate that local coordination structure is essentially the same in amorphous and crystalline complexes [7, 10].
In previous works, we investigated the thermal and the electrical behavior of complex PEO and the organic sodium trifluoroacetate salt (CF3COONa) [11]. The samples showed good thermal stability and high ionic conductivity in solid state. In the present work we study the phase behavior of a new electrolyte, PEO:CF3COOLi, which has good mechanical, thermal and electrical properties that can be used in batteries and electrochemical devices.
Experimental
The lithium trifluoroacetate salt and poly (ethylene oxide) (Aldrich) were purchased and dissolved independently in acetonitrile at a temperature of 348 K. The resulting solution were mixed in a stirrer for several hours and then poured into Teflon vessels where the solvent was evaporated under a dry atmosphere (desiccator with silica gel). After two weeks we got transparent, uniform and smooth polymeric membranes with thickness of 0.1mm.
The thermal characterization was carried out using a modulated differential calorimetry at a temperature rate of 10 K min-1. The measurement were done in the 281-423 K temperature range for a first run with an isothermal at 423 K and then heated from 281 K to 494 K for a second scan. The thermal treatment is used to enhance the contact between the sample and the pans, as well for dehydration of it.
Results and discussion
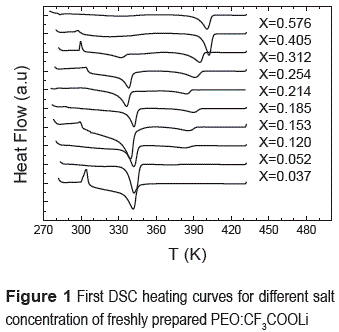
Figure 1 shows the first heating DSC curves for freshly prepared samples of PEO:CF3COOLi with various concentrations which were first quenched to 270 K and then heated. Notice the low temperature exothermic peak for some concentrations of salt (x = 0.405, 0.254, 0.150, 0.037) which is due to the cold crystallization of the corresponding polymer blend which is frequently shown when a polymer is quenched below room temperature [12].
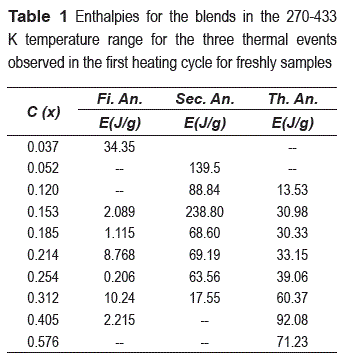
The table 1 shows the enthalpies (E) of the three anomalies, first anomaly (Fi. An.), second anomaly (Sec. An.) and third anomaly (Th. An.). Notice that when the enthalpies of the second anomaly decrease, the third anomaly increase.
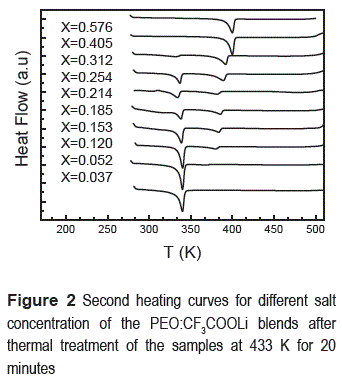
Figure 2 shows the second heating DSC curves, whose thermal events remained unaltered on subsequent runs. It measurement was taken after keeping the sample at 423 K during 20 minutes and then slowly cooled to 281 K. Notice that the first exothermic pick, does not appear after the thermal treatment. The endothermic peak observed for samples with very low salt concentration (x<0.1) about 335 K corresponds to the melting point of PEO. Notice that for x = 0.405, the endothermic peak for the melting point of PEO has vanished completely indicating that this phase is no longer present in the polymer.
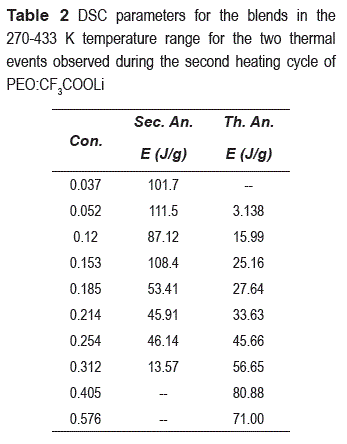
On the other hand, in figure 2 a first endothermic peak is observed at T ≈ 393 K for higher concentrations than x 0.052 whose enthalpies are sowed in the table 2.
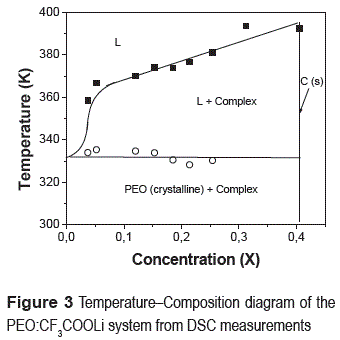
A temperature composition diagram for the PEO:CF3COOLi system is presented in figure 3 as a result of the second DSC scans.
The phase diagram obtained by DSC thermograms shows three regions with combination of different phases: when the temperature is below 330 K, PEO and complex PEO:CF3COOLi crystalline are found, when the temperature is above 330 K, liquid (likely PEO) and complex coexist. In the region of low concentrations is possible to find only liquid, however at highest temperature, the liquid phase is the only one present. The DSC results show evidence for a crystalline complex in the PEO:CF3COOLi system for concentration ofx = 0,405, whose melting point is about 392 K. The DSC results then show evidence for a crystalline complex in the PEO-CF3COOLi system, corresponding a mass fraction concentration of x = 0,405, whose melting point is at about 392 K with an enthalpy of about 81 J/g.
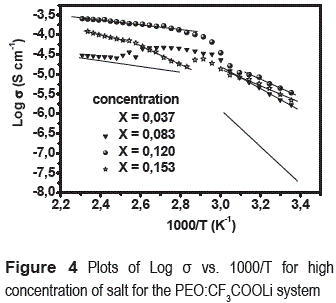
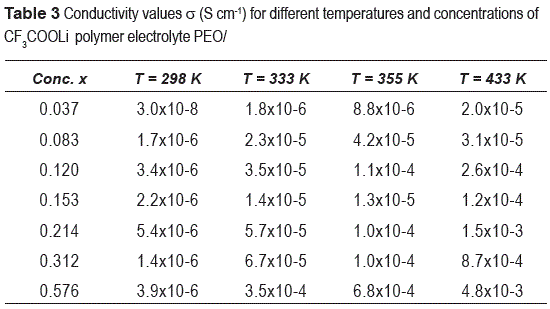
Figure 4 shows the Arrhenius plots, logσ vs. 1000/T for dried samples with different salt concentrations; strong variation of conductivity is observed in a temperature range of 298-433 K. The highest conductivity was obtained for the highest salt concentration as a result of the increase of the density of charge carriers. Some values of conductivity for different concentrations of salt are show in the table 3.
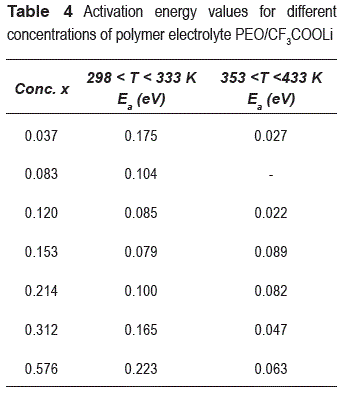
The activation energy values obtained by a fit to Arrhenius relation (σ=σ0 exp(-Ea/kT)), are show in the table 4. We can see two different regions: 298 K < T < 333 K (low temperatures region) and 353 K < T < 433 K (high temperatures region) with Arrhenius behavior; between these regions there is a transition region, which is in agree whit melting point of crystalline PEO observed with DSC.
Conclusions
We have obtained new polymer electrolytes membranes based on the PEO:CF3COOLi system with good mechanical, thermal and electrical properties for potential application in all solid rechargeable alkaline batteries.
A phase diagram obtained by DSC thermograms, it shows three regions with combination of amorphous ad crystalline phases.
The change of phases shown with DSC data as a function of the salt content and the high conductivity in the blends support the formation of the crystalline complex in this polymeric system, with best values of conductivity at height concentrations of salt.
Acknowledgement
The authors are grateful for the financial support from the Comité Central de Investigaciones Universidad del Tolima, the Centro de Excelencia en Nuevos Materiales, CENM, International Science Program of the Uppsala University, Sweden and COLCIENCIAS.
References
1. V. Di Noto, S. Lavina, G. Giffin, E. Negro, B. Scrosati. ''Polymer Electrolytes: Present, past and future''. Electrochimica Acta. Vol. 57. 2011. pp. 4-13. [ Links ]
2. M. Nadherna, F. Opekar, J. Reiter, K. Stulik. ''A planar, solid-state amperometric sensor for nitrogen dioxide, employing an ionic liquid electrolyte contained in a polymeric matrix''. Sensors and Actuators B: Chemical. Vol. 161. 2012. pp. 811-817. [ Links ]
3. A. Dey, S. Karan, A. Dey, S. De. ''Structure, morphology and ionic conductivity of solid polymer electrolyte''. Materials Research Bulletin. Vol. 46. 2011. pp. 2009-2015. [ Links ]
4. S. Jin, Y. Chan, Y. Kook. ''Ionic conductivities of solid polymer electrolyte/salt systems for lithium secondary battery''. Polymer. Vol. 46. 2005. pp. 3111-3118. [ Links ]
5. H. Aydin, M. Senel, H. Erdemi, A. Baykal, M. Tulu, A. Ata, A. Bozkurt. ''Inorganic-organic polymer electrolytes based on poly(vinyl alcohol) and borane/poly(ethylene glycol) monomethyl ether for Li-ion batteries''. Journal of Power Sources. Vol. 196. 2011. pp. 1425-1432. [ Links ]
6. J. Ah, J. Koh, D. Kyu, J. Hak. ''Preparation and characterization of crosslinked proton conducting membranes based on chitosan and PSSA-MA copolymer''. Solid State Ionics. Vol. 180. 2009. pp. 998-1002. [ Links ]
7. Y. Andreev, P. Bruce. ''Polymer electrolyte structure and its implications''. Electrochimica Acta. Vol. 45. 2000. pp. 1417-1423. [ Links ]
8. Q. Zhang. Investigating Polymer Conformation in Poly (Ethylene Oxide) (PEO) Based Systems for Pharmaceutical Applications. Master of Science Thesis. Department of Applied Physics. Chalmers University of Technology. Goteborg, Sweden. 2011. pp. 4-11. [ Links ]
9. A. Vallée, S. Besner, J. Prud'homme. ''Comparative study of poly(ethylene oxide) electrolytes made with LiN(CF3SO2)2, LiCF3SO3 and LiClO4: Thermal properties and conductivity behaviour''. Electrochimica Acta. Vol. 37. 1992. pp. 1579-1583. [ Links ]
10. J. Dygas, B. Misztal, Z. Florjanczyk, F. Krok, M. Marzantowicz, E. Zygadlo. ''Effects of inhomogeneity on ionic conductivity and relaxations in PEO and PEO-salt complexes''. Solid State Ionics. Vol. 157. 2003. pp. 249-256. [ Links ]
11. J. Castillo, I. Delgado, M. Chacón, R. Vargas. ''New solid ionic conductor based on poly(ethylene oxide) and sodium trifluoroacetate''. Electrochimica Acta. Vol. 46. 2001. pp. 1695-1697. [ Links ]
12. T. Hatakeyma, H. Kasuga, M. Tanaka, H. Hatakeyama. ''Cold crystallization of poly(ethylene glycol)-water systems''. Thermochimical Acta. Vol. 465. 2007. pp. 59-66. [ Links ]