Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230
Rev.fac.ing.univ. Antioquia no.63 Medellín Apr./June 2012
ARTÍCULO ORIGINAL
Structural modification of trans-cinnamic acid using Colletotrichum acutatum
Modificación estructural de ácido trans-cinámico empleando Colletotrichum acutatum
Rodrigo Velasco B.1, Jesús H. Gil G.1,2, Carlos M. García P.1, Diego L. Durango R.1
1Grupo de Química de los Productos Naturales y los Alimentos. Facultad de Ciencias. Escuela de Química. Universidad Nacional de Colombia. Calle 59a 63-020 Autopista Norte. AA 3840. Medellín, Colombia.
2Departamento de Ingeniería Agrícola y Alimentos. Facultad de Ciencias Agropecuarias. Universidad Nacional de Colombia. Calle 64 x Carrera 65 Autopista Norte. AA 3840. Medellín, Colombia.
*Autor de correspondencia: teléfono: + 57 + 4 + 430 93 92, fax: + 57 + 4 + 260 44 89, correo electrónico: dldurango@unal.edu.co (D. Durango)
(Recibido el 18 de febrero de 2011. Aceptado el 23 de mayo de 2012)
Abstract
The biotransformation of trans-cinnamic acid by whole cells of the Colombian native phytopathogenic fungus Colletotrichum acutatum was studied. Initially, fungitoxicity of this compound against C. acutatum was evaluated; trans-cinnamic acid exhibited a moderate to weak toxicity against the microorganism and apparently a detoxification mechanism was present. Then, in order to study such mechanism and explore the capacity of this fungus to biotransform trans-cinnamic acid into value-added products, the microorganism was incubated with the substrate using three different culture media (Czapeck-Dox, Sabouraud and PDB) at room conditions. Using Czapeck-Dox medium, whole cultures of C. acutatum reduced trans-cinnamic acid, first to aldehydes (trans-cinnamaldehyde and 3-phenylpropanal), then to alcohols (cinnamyl alcohol and 3-phenyl-1-propanol). Subsequently, these alcohols were transformed to the corresponding acetyl esters. Nevertheless, some of these products were absent or present at different concentration when culture medium was changed. The results suggest a mechanism of detoxification in which the α,β-unsaturated carbonyl system is affected. Besides, the formed metabolic products are useful compounds used as fragrances and flavors. Therefore, metabolism of trans-cinnamic acid using C. acutatum can provide new potential metabolic targets to control C. acutatum as well as a simple and efficient way to obtain flavor compound and perfumes, such as cinnamyl alcohol and 3-phenyl-1-propanol, and their acetyl esters.
Keywords: Biocatalyst, phytopathogenic fungus, metabolic pathway, culture media
Resumen
Se estudió la biotransformación de ácido trans-cinámico mediante células completas del hongo fitopatógeno nativo colombiano Colletotrichum acutatum. Inicialmente, se evaluó la fungitoxicidad de este compuesto contra C. acutatum; el ácido trans-cinámico exhibió una toxicidad moderada a débil contra el microorganismo y aparentemente se presentó un mecanismo de detoxificación. Luego, para estudiar tal mecanismo y explorar la capacidad de este hongo para biotransformar el ácido trans-cinámico en productos con valor agregado, el microorganismo se incubó con el sustrato usando tres medios de cultivo diferentes (Czapeck-Dox, Sabouraud y PDB) a condiciones ambientales. Usando el medio Czapeck-Dox, los cultivos completos de C. acutatum redujeron el ácido trans-cinámico, primero a aldehídos (trans-cinamaldehido y 3-fenilpropanal), luego a alcoholes (alcohol cinamílico y 3-fenil-1-propanol). Posteriormente, estos alcoholes fueron transformados a los correspondientes ésteres de acetilo. Sin embargo, algunos de estos productos estuvieron ausentes o presentes a una concentración diferente cuando se cambió el medio de cultivo. Los resultados sugieren un mecanismo de detoxificación en el cual el sistema carbonílico α,β-insaturado es afectado. Por otra parte, los productos metabólicos formados son compuestos útiles usados como fragancias y sabores. Por consiguiente, el metabolismo del ácido trans-cinámico usando C. acutatum puede proporcionar nuevos blancos metabólicos para controlar C. acutatum así como también una forma simple y eficiente para obtener sabores y perfumes, tales como el alcohol cinamílico y el 3-fenil-1-propanol, y sus ésteres de acetilo.
Palabras clave: Biocatalizador, hongo fitopatógeno, ruta metabòlica, medios de cultivo
Introduction
Biocatalysis or biotransformation encompasses the use of biological systems to catalyze the conversion of one compound to another. The catalyst part can thereby consist of whole cells, cellular extracts, or isolated enzyme(s). If the conversion is developed by a free and/or immobilized enzyme, it means biocatalysts, but if these transformations take place by the whole cell (with the correct enzyme) we talk about biotransformation [ 1] . Although the current interest in applying biotransformations in organic synthesis is mainly related to the preparation of enantiopure compounds, these can also used to perform transformations of achiral functional groups. The reason is that biotransformations are carried out usually at room temperature and atmospheric pressure, avoiding the use of extreme reaction conditions, and minimizing problems of isomerization, racemization, epimerization or transposition [ 2] . Therefore, biotransformations attract considerable attention due to its simple, cheap and benign methodologies that combines green chemistry with high efficiency [ 3] . Besides, biotransformation experiments using phytopathogenic fungi provide information on the detoxification mechanism used by these microorganisms and give an indication of the structural modifications that may be necessary if substrates of this type are to be further developed as selective fungal control agents [ 4] .
On the other hand, phenylpropenoides and cinnamates can potentially serve as a good source of starting material for the production of value-added compounds. Several studies have demonstrated that valuable aroma and flavoring compounds, and pharmaceutical intermediates, are produced as intermediates in the degradation pathways of such phenylpropenoides and cinnamates [ 5,6] . Thus, biotransformation of these compounds seems to be a reasonable alternative to produce raw materials for different industries. Also, products of such bioconversions are considered natural [ 7] , which gives them better perspectives of use than synthetic counterparts.
In addition, several phenylpropenoides and cinnamates have been reported possessing antifungal activity [ 8,9] and have been suggested to be effective to control postharvest pathogens [ 9] . Nevertheless, knowledge about microbial metabolism of these compounds by phytopathogenic fungi is still limited. Understanding potential biofungicide metabolism in microorganisms is necessary for fungicide development as well as for safe and efficient use.
In this sense, filamentous phytopathogenic fungi have high potential for the biotransformation of compounds with aromatic structure; however, cellular pathways and metabolic processes involved must be known better. This paper reports for the first time the capability of the fungus C. acutatum, a cosmopolitan filamentous phytopathogenic fungus to biotransform trans-cinnamic acid into value-added products. A possible metabolic pathway of the biotransformation and culture medium effect is also discussed.
Experimental
Analytical methods
Thin layer chromatography (TLC) was made on precoated plates (Si 60 F254, 0.25 mm, Merk). Mixtures of n-hexane:EtOAc were used as mobile phase. Column chromatography (CC) employed silice gel 60 (Merck) and Sephadex LH-20. Gas chromatography (GC) was performed on a Hewlett-Packard 6890 (Agilent Technologies) gas chromatograph coupled with a HP 5973 MSD with a HP-5 column (30 m x 0.25 mm i.d.; coating thickness 0.25 µm). Chromatographic conditions were: column temperature, 50-250°C at 10°C/min and keep it five minutes; injector temperature, 150°C; detector temperature, 280°C; carrier gas, N2 at 1 mL/min. Relative composition of the individual constituent was determined from the peaks average area. EI- MS measurements were obtained using gas chromatography-mass spectrometry (GC-MS). Substances were identified by comparison of their spectroscopic properties with those of reference substances and by comparison with the NIST 2002 Mass Spectral Library.
Biological and chemical materials
C. acutatum strain was provided by the Laboratory of Phytopathology (Universidad Nacional de Colombia-Medellín). The fungus was isolated from diseased Solanum betaceum cav. Sendt (tamarillo) fruits, and characterized through morphological and molecular data by Dr. Afanador-Kafuri. The fungus was maintained in a Potato Dextrose Agar (PDA) medium at 24±2°C, and monthly subcultured in Petri dishes. To evaluate the antifungal activity, previously sterile Petri dishes measuring 15 cm in diameter were inoculated with 1 mL of a spore suspension of the fungus. The suspension was uniformly spread over the medium using a bent glass rod. After that, the inoculated medium was incubated at 25°C for 48 h. A mycelial disc of 5 mm of diameter was used for antifungal test. The substrate for biotransformation, trans--cinnamic acid (A), was purchased from Alfa Aesar, and compounds cinnamyl alcohol (B) and 3-phenyl-1-propanol (C) were obtained from Sigma-Aldrich Co. Bacto Agar was obtained from Becton, Dickinson and Co. Yeast extract was from Oxoid Ltd. Peptone from casein (pancreatically digested) was acquired from Merck KGaA.
Antifungal bioassay
In order to investigate the toxicity of (A) against C. acutatum, the poisoned food technique described by Velasco et al. [ 6] was used. Different concentrations (50-700 µg/mL) of (A) dissolved in acetone (2 µL/mL) were diluted in Petri dishes with PDA. All concentrations were tested in triplicate, and the results are shown as mean values of colony diameters [ ± standard deviation (SD)] . Petri dishes with acetone were used as the control. The Petri dishes were incubated at room temperature and the diameter of the mycelial growth was measured each 24 hours. The incubation was stopped when the mycelial mass of control Petri dishes had almost filled it (ca. 288 h). The relative growth inhibition of the treatment compared to the control was calculated as percentage, using the formula: Inhibition (%) = {1 - radial growth of treatment (mm)/radial growth of control (mm)}x 100 (1)
Preculture of C. acutatum
The microorganism was inoculated into 1.0 L Erlenmeyer flasks, containing 500 mL of Czapeck-Dox liquid medium. Erlenmeyer flasks were shaken (reciprocating shaker, 120 rpm) at room temperature for 168 h. Mycelia were recovered by filtration, and washed with H2O to inoculate in a new culture medium with the substrate for the biotransformation and time- course experiments.
Preparative biotransformation
Mycelium of C. acutatum was transplanted into four 1.0 L Erlenmeyer flask containing 500 mL of sterilized Czapeck-Dox culture medium and the substrate (at 400 µg/mL). Cultivation and biotransformation was carried out at room temperature and stirring (120 rpm) for 336 h. After the incubation period, culture medium and mycelia were separated by filtration. Mycelia were discarded and culture medium was used to isolate the metabolic products. Control was carried out in order to verify the presence of similar compounds on the fungus culture (without substrate).
Isolation and identification of metabolic products
The culture medium was saturated with NaCl, refrigerated, filtered and extracted with CH2Cl2 (3x2.0 L). Afterward, the medium was acidified to pH 2 with 1.0 M HCl, and extracted again with CH2Cl2 (2x2.0 L). Both organic extracts were mixed, dried over anhydrous Na2SO4 and concentrated in vacuum, and the crude extract was chromatographed on a silice gel column. Elution was performed with an n-hexane-EtOAc gradient system. Several fractions were collected and separated into 4 groups (I-IV) according to TLC profiles. Fractions II and III were fractionated by size-exclusion column chromatography over Sephadex LH-20 (100x2 cm) using n-hexane-CH2Cl2-MeOH (50:25:25, v/v) as eluent to yield metabolic compounds (B) and (C); these compounds were isolated and identified by spectroscopic analysis, and comparison with authentic samples. Spectral data and retention times of (B), (C), (D), and (E) are in good agreement with those observed in the literature [ 5, 6] , corresponding to cinnamyl alcohol, 3-phenyl-1-propanol, cinnamyl acetate, and 3-phenyl propyl acetate, respectively. In addition, many minor metabolites were detected.
Time-course experiments and effect of culture medium
Portions of 1 mL of the mycelia were transferred to inoculate seven 500 mL flasks, each containing 125 mL of Czapeck-Dox medium and the substrate (A). Cultivation was carried out at room temperature and stirring (reciprocating shaker, 120 rpm) for 336 h. The culture medium from each flask was removed every 48 hours and then, it was saturated with NaCl, refrigerated, filtered and extracted with CH2Cl2 and the solvent was subsequently evaporated. These extracts were analyzed by TLC and GC-MS. The ratios between the substrate and metabolic products were determined on the basis of GC peak areas. Control cultivation with no substrate was also performed. Further, time-course experiments using Sabouraud and PDB liquid media were carried out. Cultivation and analyses were performed under the same conditions described for the Czapeck-Dox medium.
Results and discussion
Fungitoxicity bioassay
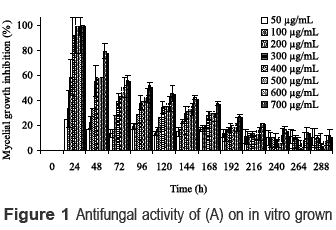
In order to determine the concentration to use in the biotransformation process, the toxicity of (A) against C. acutatum was examined. Overall, compound (A) displayed a moderate to weak activity against the fungus, as can be seen in figure 1. The inhibition of C. acutatum growth was depending of concentration. Complete inhibition activity of C. acutatum on exposure to (A) for 24 h was observed from 300 to 700 µg/mL. However after that period, the inhibitory effect was strongly decreased at all the concentrations evaluated.
Cheng et al. [ 10] have reported that (A) shows a remarkable antifungal activity against L. sulphureus and L. betulina, with IC50 values of 87.4 and 55.8 µg/mL, respectively. Authors suggested that the acid group and the conjugated double bond are important features to exhibit the strong antifungal action. A similar observation was also noted by different authors in previous studies [ 11,12] .
As shown in figure 1, the inhibitory effect of (A) diminished with time, a fact that suggests that the fungus has a detoxification mechanism. In order to study this mechanism and explore the biotechnological potential of C. acutatum to biotransform (A) into value-added products, the microorganism was incubated with the substrate at 400 µg/mL during 336 h. Such concentration was able to inhibit nearly 90% of fungal growth for 24 h, retaining an inhibition percentage of about 40% after 120 h.
Isolation and identification of metabolic products
To isolate the main metabolic products, a preparative incubation of (A) in Czapeck-Dox liquid medium using C. acutatum was performed. During biotransformation, a pleasant, sweet and floral odor was perceived, indicating the presence of aroma compounds. A comparison through TLC and GC among the extract obtained from the biotransformation and the control, showed that C. acutatum transformed (A) into various metabolites. Two metabolic products (B) and (C) were isolated, and their structures were elucidated on the basis of spectral data, corresponding to cinnamyl alcohol, and 3-phenyl-1-propanol, respectively. Lower amounts of (D) and (E) were observed; these compounds were detected by means of TLC and GC analysis, and their mass spectral and retention times were consistent with those reported to cinnamyl acetate and 3-phenyl propyl acetate, respectively. Additionally, other minor metabolites were detected.
It is noteworthy that C. acutatum was able to reduce the carboxylic group without affect the double bond to give the major product, cinnamyl alcohol (B). This compound is valuable in perfumery for its odor and fixative properties. It is a component of some flower compositions (lilac, and hyacinth); in aromas is used for cinnamon notes and for rounding of fruit aromas [ 13] . Otherwise, reduction of carboxylic acid to aldehydes and subsequently alcohols is, biologically, a difficult process due to the very low redox potential required for the reaction (-600 mV) [ 14] . For this reason, the reduction of non-activated carboxylic acids has been described only for a limited number of mesophilic microorganisms. Nocardia [ 15] , Clostridium formicoaceticum [ 16] , and some fungi [ 17] reduce aromatic carboxylic acids to alcohols. C. formicoaceticum [ 16] also reduce aliphatic carboxylic acids. Therefore, biocatalytic reductions of carboxylic acids are attractive and constitute a good alternative to chemical methods. In general, chemical methods for carboxylic acid reductions are limited, and they usually require prior derivation and product deblocking with reactants containing functional groups [ 18] .
The biotransformation of (A) by C. acutatum also allowed the isolation of 3-phenyl-1-propanol (C), resulting from the reduction of the double bond C-C and the carbonyl group. Metabolite (C) was the second major metabolite through the bioprocess. It has a sweet, balsamic and floral odor and is also used as a cosmetic and perfume ingredient [ 13] . Simultaneously, alcohols (B) and (C) were esterified by the microorganism to generate the corresponding acetates (D) and (E). These esters have been employed as block of construction of flavors and are widely used in the production of perfumes [ 13] . Compound (D) occurs in cassia oil and is a colorless liquid with a sweet-flowery-fruity, slightly balsamic odor.
In addition, recent studies about the structure- antifungal activity relationship of cinnamaldehyde congeners have shown that compounds having an aldehyde group or an acid group, and a conjugated double bond, possesses much stronger antifungal activity [ 10] . The authors reported that (B) and (D) were less inhibitory to fungal growth than (A) against L. betulina and L. sulphureus at the concentration of 100 µg/mL. Thus, the formation de (B), (C), (D) and, (E) from (A) by C. acutatum suggests a mechanism of detoxification in which the α,β-unsaturated carbonyl system is affected. Indeed, the MIC value obtained at 24 h of incubation (defined as the lowest compound concentration exhibiting approximately 50% reduction of growth compared with the control) for (A), gave the lowest value followed by (B) and (C), respectively (Data not shown). Therefore, (A) was the most active against C. acutatum, followed by (B) and (C). Based on these findings, it is possible to postulate that one of the possible modes of action of (A) may be due to its role as Michael-type acceptor for biological nucleophiles. In contrast, compounds lacking the α,β-unsaturated carbonyl system, such as (B), (C), were found to be less active.
Time-course experiments and influence of the culture medium
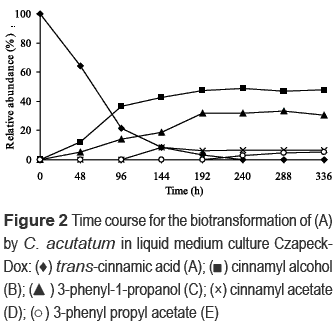
In this test, (A) was incubated with the microorganism during 336 h. Every 48 h, the medium from one flask was removed and extracted, and then analyzed through TLC and GC. All the metabolic products and the substrate were quantitatively measured through GC. As it is shown in figure 2, (A) was mainly transformed into (B). After 144 h about 90% of (A) was modified. Under the conditions used, the alcohol (B) reached about 47% of the products in 192 h and continued stable until the end of process. In the same way, the alcohol (C) reached about 31% at 192 h, and then its concentration remained almost unchanged. The increase in relative abundance of (B) and (C) coincided with the decline of (A). Additionally, the metabolic compounds (D) and (E) increased slowly after 96 and 192 h, respectively, but no one of these metabolites obtained a considerable concentration at the end of the evaluation (only 7 and 6% in the order given).
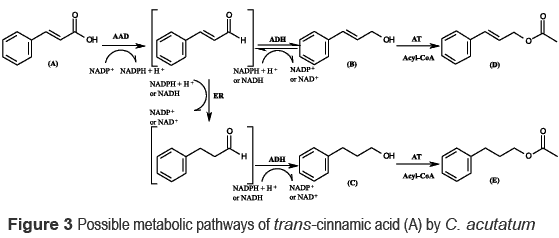
The metabolic pathways for the biotransformation of (A) by C. acutatum were proposed according to the time course experiment and the structures of the metabolites identified. Whole cultures of C. acutatum reduce the carboxylic acid (A) to trans-cinnamaldehyde, then to alcohols [ (B) and (C)] and subsequently to the corresponding acetyl esters [ (D) and (E)] . The reduction reaction has been proposed to occur sequentially, and it involves at least three separate enzymes [ 19] . These enzymes are an aryl-aldehyde oxidoreductase carboxylic acid reductase, AAD that converts (A) into cinnamaldehyde, an alcohol dehydrogenase aldehyde reductase ADH that converts cinnamaldehyde into the alcohol (B), and an acyl transferase (AT) that catalyzes the formation of acetyl ester (D) from the alcohol product [ 15] . Furthermore, the reduction of the double bond from trans-cinnamaldehyde, via 3-phenylpropanal (formed by the action of the enoate reductase, ER), and further overreduction of the saturated aldehyde leads finally to the formation of the saturated alcohol (C) [ 20] , as described in figure 3. Although the formation of (C) from (B) by C. acutatum has been recently reported, such process results of the reversibility of the interconversion trans-cinamaldehyde-cynnamilic alcohol [ 21] , through the sequence [ (B)-(trans-cinnamaldehyde)-(3- phenylpropanal)-(C)] . Finally, alcohols (B) and (C) were acetylated through esterification by acyl transferases (AT) to produce (D) and (E).
It is noteworthy that during the conversion of (A) by C. acutatum no aldehydes were detected. This phenomenon was also observed for the reduction of acids by, e.g. C. thermoaceticum [ 22] , N. asteroides JCM 3016 [ 23] , and several fungi [ 17] . However, Correa et al. [ 5] recently reported that trans-cinnamaldehyde, the intermediate of the reduction of (A), was quickly transformed to (B) and (C) by C. acutatum. This indicates that the fungus was able to reduce the aldehyde to the alcohol, and saturate the double bond. In this sense, the lack of aldehydes during biotransformation of (A) by C. acutatum indicated that the second reaction, the reduction of trans-cinnamaldehyde, was faster than the reduction of (A). Therefore, it is very likely that for (A), the reduction to aldehyde is the rate-limiting step of the conversion of acids to alcohols. Moreover, the low accumulation of aldehydes in the culture medium is probably due to its higher cell toxicity [ 24] . In fact, cinnamaldehyde has been recognized by displaying antibacterial and antifungal properties [ 25] , including against C. acutatum [ 5] .
As can be seen from figure 3, the intermediate trans-cinnamaldehyde is reduced through two pathways: (1) reduction of the aldehyde to the allylic alcohol (which is not a substrate for enoate reductases) by the action of an alcohol dehydrogenase (ADH) and the coenzymes NADH and NADPH [ 26] , and (2) saturation of the C=C bond by an ER to furnish the saturated aldehyde. Overall, the chemoselectivity in the bioreductions of the bonds C=C versus C=O by whole cells of C. acutatum is poor, which is due to the presence of competing enzymes [ 6] . Because enoate reductases and alcohol dehydrogenases depend on the same nicotinamide cofactor, redox-decoupling of both enzyme activities is hardly possible [ 20, 26] . However, the formation of (B) as the major metabolite throughout the process suggests that the conversion [ (trans-cinnamaldehyde) to (B)] is slightly faster than [ (trans-cinnamaldehyde) to (C)] .
The ability to C. acutatum to reduce (A) is an interesting aspect, due to that microbiological reduction of carboxylic acids is an unusual and potentially useful biocatalytic reaction, which has not yet been widely examined and exploited [ 27] . This article is the first report on the capacity of C. acutatum to reduce a carboxylic acid to value-added products. Nevertheless, knowledge about the properties of each enzyme involved in serial reactions is essential to establishing useful whole-cell biocatalytic processes.
Due that the efficient conversion of carboxylic acids to alcohols or their corresponding acetyl esters is an attractive whole-cell reaction sequence for the biocatalytic synthesis of fragrances and flavors, a complementary study of the culture medium effect on biotransformation of (A) was carried out. Therefore, (A) was incubated with the microorganism using PDB and Sabouraud media. Biotransformation on PDB showed to be slower than on Czapeck-Dox medium; substrate (A) was gradually converted (about 70% after 240 h), mainly to metabolites (B) and (C). During the first 192 h, traces of these alcohols were detected. Then, relative abundances of (B) and (C) increasing to about 25% at 288 h and remained constant until 336 h. It seems remarkable that in PDB medium, compounds (D) and (E) were not detected. Instead, other metabolites (e.g. phenylacetic acid and benzaldehyde) were found at very low relative abundances (<5%). Additionally, the biotransformation using Sabouraud demonstrated to be faster than on Czapeck-Dox medium. Substrate (A) was rapidly converted by C. acutatum (>95% after 96 h). Metabolite (C) was the main metabolic product throughout the process; it presented a relative abundance of 50% at 144 h. However, at 240 h and after, (C) was not detected. Under the conditions used, the alcohol (B) was only found at 216 h, reaching a relative abundance of approximately 5%. Similar to biotransformation of (A) by C. acutatum on PDB, the compounds (D) and (E) were absent on Sabouraud. Instead, three compounds with a molecular ion of 154 amu, corresponding to hydroxilated derivatives of (C), were found. It seems remarkable that in Sabouraud medium, the conversion of (A) by the fungus was more selective and slower toward the formation of (C) than in Czapeck-Dox. Also, it seems noteworthy that some minor metabolites detected on Sabouraud and PDB were not detected on Czapeck-Dox.
Such specificity of the medium for transformation has also been previously reported [ 28, 6] . Authors suggest that the difference in the compounds production in each medium means that the enzymes presents in the microorganism are induced in different way, due to the suitability of each medium for the production of some specific metabolites. Thus, the Czapeck-Dox medium, rich in minerals, could be providing metal ions needed to some catalytic processes (such as cofactors or Lewis acids), favoring certain stages involving the ADH and Acyl-transferases. In addition, the culture medium possibly influences the physiological status of the fungus, which in turn, could induce differences in efficiency towards the formation of some products. However, further investigations are needed to determine how the composition of culture medium affects the enzymatic behavior.
Conclusions
In conclusion, according to the results described herein, the trans-cinnamic acid presents a moderate to weak antifungal activity against C. acutatum. Also, a detoxification mechanism was established. Results obtained from biotransformation experiments demonstrate the ability of the phytopathogenic fungus to transform trans-cinnamic acid. Thus, using Czapeck-Dox medium, two compounds were isolated and identified: cinnamyl alcohol, and 3-phenyl-1-propanol, and two products were also detected by GC: cinnamyl acetate, and 3-phenyl propyl acetate. Therefore, C. acutatum was able to reduce trans-cinnamic acid, first to aldehydes, and then to alcohols. Further, these alcohols were transformed to the corresponding acetyl esters. It suggests a mechanism of detoxification in which the α,β-unsaturated carbonyl system is modified. Interestingly, the products formed are valuable aroma and flavoring compounds, which opens good prospects for production of these through biotechnological processes using C. acutatum. Unfortunately, using PDB and Sabouraud media, some of these products were absent or present at a lower concentration. Besides, others metabolites were detected. Further investigations are needed to produce these metabolites in large quantities by improving the conditions of the biotransformation.
Acknowledgements
Special thanks to DIME (Dirección de Investigación Sede Medellín) and Universidad Nacional de Colombia for their financial support.
References
1. J. Leresche, H. Meyer. ''Chemocatalysis and biocatalyst (biotransformation): some thoughts of a chemist and of a biotechnologist''. Org. Process. Res. Dev. Vol. 10. 2006. pp. 572-580. [ Links ]
2. H. Luna. ''Aplicación de la biocatálisis a la preparación de intermediarios para la síntesis de fármacos''. J. Mex. Chem. Soc. Vol. 48. 2004. pp. 211-219. [ Links ]
3. K. Faber, R. Patel. ''Chemical biotechnology: A happy marriage between chemistry and biotechnology: asymmetric synthesis via green chemistry''. Curr. Opin. Biotechnol. Vol. 11. 2000. pp. 517-519. [ Links ]
4. M. Daoubi, R. Galán, A. Benharref, I. Collado. ''Screening study of lead compounds for natural product-based fungicides: antifungal activity and biotransformation of 6a, 7a-Dihydroxy-P-himachalene by Botrytis cinerea''. J. Agric. Food Chem. Vol. 53. 2005. pp. 6673-6677. [ Links ]
5. Y. Correa, D. Durango, C. García. ''Transformación microbiana del arilpropanoide cinamaldehído con el hongo fitopatógeno Colletotrichum acutatum''. Vitae. Vol. 16. 2009. pp. 83-91. [ Links ]
6. R. Velasco, J. Gil, C. García, D. Durango. ''Production of 2-phenylethanol in the biotransformation of cinnamyl alcohol by the plant pathogenic fungus Colletotrichum acutatum''. Vitae. Vol. 17. 2010. pp. 272-280. [ Links ]
7. E. Shimoni, U. Ravid, Y. Shoham. ''Isolation of a Bacillus sp. capable of transforming isoeugenol to vanillin''. J. Biotechnol. Vol. 78. 2000. pp. 1-9. [ Links ]
8. S. Zacchino, S. López, G. Pezzenati, R. Furlán, C. Santecchia, L. Muñoz, F. Giannini, A. Rodríguez, R. Enriz. ''In vitro evaluation of antifungal properties of phenylpropanoids and related compounds acting against dermatophytes''. J. Nat. Prod. Vol. 62. 1999. pp. 1353-1357. [ Links ]
9. D. Sivakumar, R. Wijeratnam, R. Wijesundera, M. Abeyesekere. ''Control of postharvest diseases of rambutan using cinnamaldehyde''. Crop Prot. Vol. 21. 2002. pp. 847-852. [ Links ]
10. S. Cheng, J. Liu, E. Chang, S. Chang. ''Antifungal activity of cinnamaldehyde and eugenol congeners against wood-rot fungi''. Bioresource Technol. Vol. 99. 2008. pp. 5145-5149. [ Links ]
11. S. Chang, P. Chen, S. Chang. ''Antibacterial activity of leaf essential oils and components from Cinnamomum osmophloeum''. J. Ethnopharmacol. Vol. 77. 2001. pp. 123-127. [ Links ]
12. H. Lee, S. Cheng, S. Chang. ''Antifungal property of the essential oils and their constituents from Cinnamomum osmophloeum leaf against tree pathogenic fungi''. J. Sci. FoodAgric. Vol. 85. 2005. pp. 2047-2053. [ Links ]
13. H. Surburg, J. Panten. Common fragrance and flavor materials: Preparation, Properties and Uses. 5th ed. Ed. Wiley-VCH Verlag Gmbh. Weinheim (Germany). 2006. pp. 7-175. [ Links ]
14. R. Thauer, K. Jungermann, K. Decker. ''Energy conservation in chemotropic anaerobic bacteria''. Bacteriol. Rev. Vol. 41. 1977. pp. 100-180. [ Links ]
15. Y. Chen, J. Rosazza. ''Microbial transformation of ibuprofen by a Nocardia species''. Appl. Environ. Microbiol. Vol. 60. 1994. pp. 1292-1296. [ Links ]
16. L. Fraisse, H. Simon. ''Observations on the reduction of non-activated carboxylates by Clostridium formicoaceticum with carbon monoxide or formate and the influence of various viologens''. Arch. Microbiol. Vol. 150. 1988. pp. 381-386. [ Links ]
17. H. Arfmann, W. Abraham. ''Microbial reduction of aromatic carboxylic acids''. Z. Naturforsch. Vol. 48c. 1993. pp. 52-57. [ Links ]
18. A. He, T. Li, L. Daniels, I. Fotheringham, J. Rosazza. ''Nocardia sp. carboxylic acid reductase: cloning, expression, and characterization of a new aldehyde oxidoreductase family''. Appl. Environ. Microbiol. Vol. 70. 2004. pp. 1874-1881. [ Links ]
19. T. Li, J. Rosazza. ''The carboxylic acid reduction pathway in Nocardia. Purification and characterization of the aldehyde reductase''. J. Industrial Microbiol. Biotechnol. Vol. 25. 2000. pp. 328-332. [ Links ]
20. M. Hall, B. Hauer, R. Stuermer, W. Kroutil, K. Faber. ''Asymmetric whole-cell bioreduction of an α,β- unsaturated aldehyde (citral): competing prim-alcohol dehydrogenase and C-C lyase activities''. Tetrahedron: Asymmetr. Vol. 17. 2006. pp. 3058-3062.
21. R. Stuermer, B. Hauer, M. Hall, K. Faber. ''Asymmetric bioreduction of activated C=C bonds using enoate reductases from the old yellow enzyme family''. Curr. Opin. Chem. Biol. Vol. 11. 2007. pp. 203-213. [ Links ]
22. H. Simon, H. White, H. Lebertz, J. Thanos. ''Reduktion von 2-enoaten und alkanoaten mit kohlenmonoxid oder formiat, viologenen und Clostridium thermoaceticum zu gesättigten säuren und ungesättigten bzw, gesättigten alkoholen''. Angew. Chem. Vol. 99. 1987. pp. 785-787. [ Links ]
23. N. Kato, H. Konishi, K. Uda, M. Shimao, C. Sakazawa. ''Microbial reduction of benzoate to benzyl alcohol''. Agric. Biol. Chem. Vol. 52. 1988. pp. 1885-1886. [ Links ]
24. R. Villa, F. Molinari. ''Reduction of carbonylic and carboxylic groups by plant cell cultures''. J. Nat. Prod. Vol. 71. 2008. pp. 693-696. [ Links ]
25. D. Sivakumar, R. Wijeratnam, R. Wijesundera, M. Abeyesekere. ''Control of postharvest diseases of rambutan using cinnamaldehyde''. Crop Prot. Vol. 21. 2002. pp. 847-852. [ Links ]
26. J. Carballeira, M. Quezada, P. Hoyos, Y. Simeó, M. Hernaiz, A. Alcántara, J. Sinisterra. ''Microbial cells as catalysts for stereoselective red-ox reactions''. Biotechnol. Adv. Vol. 29. 2009. pp. 686-714. [ Links ]
27. J. Rosazza, T. Li. Carboxylic acid reductase and methods for use of the same. U.S. patent 5795759. August 18. 1998. [ Links ]
28. S. Gurram, N. Kollu, G. Sivadevuni, M. Solipuram. ''Biotransformation of albendazole by Cunninghamella blakesleeana: influence of incubation time, media, vitamins and solvents''. Iran. J. Biotechnol. Vol. 7. 2009. pp. 205-215. [ Links ]