Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Facultad de Ingeniería Universidad de Antioquia
versión impresa ISSN 0120-6230
Rev.fac.ing.univ. Antioquia no.65 Medellín oct./dic. 2012
ARTÍCULO ORIGINAL
ADM1 applications for a hybrid up-flow anaerobic sludge-filter bed reactor performance and for a batch thermophilic anaerobic digestion of thermally pretreated waste activated sludge
Aplicaciones de ADM1 al funcionamiento de un rector híbrido anaerobio de flujo ascendente de lecho y con filtro de lodos y a la digestión anaerobia termófila en batch de lodos activados residuales con pre-tratamiento térmico
Iván Ramírez*
Electronics Engineering Program. Faculty of Engineering. Universidad Del Quindío. Cra. 15, Calle 12 Norte. Armenia, Colombia.
*Autor de correspondencia: teléfono: + 57 + 6 +735 9359, correo electrónico: idramirez@uniquindio.edu.co (I. Ramírez)
(Recibió el 14 de julio de 2010. Aceptado el 6 de noviembre de 2012)
Abstract
The anaerobic digestion process comprises a whole network of sequential and parallel reactions of biochemical and physicochemical nature. Anaerobic digesters often exhibit significant stability problems that may be avoided only through appropriate control strategies. Such strategies require, in general, developing appropriate mathematical models aiming at understanding and optimizing the anaerobic digestion process, describing these reactions in structured manner. This work reviews the IWA Anaerobic Digestion Model No. 1 (ADM1) and discusses two model applications: the anaerobic digestion of wine distillery vinasse as substrate in a 9.8-1 hybrid UASFB reactor and batch thermophilic anaerobic digestion of thermally pretreated waste activated sludge. Predictions by the model using the parameters established in this study agreed well with the data measured under different conditions tested. The resulting models explained the dynamic evolution of the main variables, in the liquid and gas phases.
Keywords: Anaerobic digestion, modeling, ADM1, inhibition, rate-limiting step, waste activated sludge, UASFB, thermophilic, thermal pretreatment
Resumen
Los procesos de la digestión anaerobia comprenden una red completa de reacciones bioquímicas y fisicoquímicas, secuenciales y paralelas. Los digestores anaerobios a menudo exhiben importantes problemas de estabilidad que sólo pueden ser evitados a través de apropiadas estrategias de control. Tales estrategias requieren, en general, para su implementación, del desarrollo de modelos matemáticos cuya finalidad es el de permitimos mejor comprensión y optimización de los procesos de la digestión anaerobia, describiendo estas reacciones de una manera estructurada. Este trabajo revisa el modelo ADM1 de la IWA y discute dos aplicaciones del modelo: la digestión anaerobia de las aguas residuales vinazas de las destilerías de vino como sustrato en un reactor híbrido (UASFB) y la digestión anaerobia termòfila en batch de lodos activados con pre-tratamiento térmico. Las predicciones del modelo, usando los parámetros establecidos en este estudio, concuerdan bien con los resultados de las mediciones en las diferentes condiciones ensayadas. Los modelos resultantes explicaron la evolución dinámica de las principales variables, tanto en la fase liquida como la fase gaseosa.
Palabras clave: Digestión anaerobia, modelización, ADM1, inhibición, etapa de velocidad limitante, lodos activados residuales, UASFB, termófila, pre-tratamiento térmico
Introduction
Anaerobic digestion is the methanogenic fermentation of organic matter. Microorganisms metabolize organic matter in the absence of oxygen and produce biogas, which is a mixture of methane (CH4) and carbon dioxide (CO2), as well as trace gases like hydrogen sulfide (H2S) and hydrogen (H2), [1].
The anaerobic wastewater treatment process presents interesting advantages compared to the classical aerobic treatment [2]. It has a high capacity to degrade concentrated and difficult substrates (plant residues, animal wastes, food industry wastewater, and so forth), produce very little sludge, requiring little energy, and in some cases, it can even recover energy by using methane combustion. But, in spite of these advantages, anaerobic treatments plants often suffer from instability. Such instability is usually witnessed as a drop in methane production rate, a pH drop, increased volatile fatty acid (VFA) concentration, causing digester failure. This is caused by (a) feed overload, (b) feed under load, (c) entry of an inhibitor, or (d) inadequate temperature control.
The usual remedy is a rapid increase in Hydraulic Retention Time (HRT) and when this fails, the digester has to be primed with sludge from a ''healthy'' digester; however, this may be quite costly, given that anaerobic digestion is a slow process.
The most common reactor type used for wastewater anaerobic digestion is the Continuously Stirred Tank Reactor (CSTR). The main problem of this reactor type, i.e., the fact that the active biomass is continuously removed from the system leading to long retention times, has been overcome in a number of systems based on immobilization of the active biomass [3]; henceforth, referred to as high-rate systems (like biofilm rectors). Some of these reactors are: Up-fiow Anaerobic Sludge Blanket Reactor (UASBR), Expanded Granular Sludge Bed (EGSB) reactor, Anaerobic biofilter (AF), and Anaerobic Fluidized Bed Reactor(AFBR).
The term ''biofilm reactor'' refers to the class of bioreactors where the biocatalyst exists in anchored form, either on the surface of an inert ''carrier'' or attached to one another. The UASB reactor and AF are the most frequently used high-rate anaerobic reactors, but both types suffer from technical problems [4]. Granular sludge formation is the main distinguishing characteristic of UASB reactors compared to other anaerobic technologies. But with some wastewaters, granulation does not occur readily and problems can be encountered with washout of flocculent biomass [5], In a UASB reactor, very low flow rate liquid superficial velocity may cause channeling of wastewater through the bed and, therefore, poor water-sludge contact, which leads to low treatment efficiencies. In fully packed anaerobic filters, long-term operation may result in excessive biomass entrapment in the interstitial cavities in the matrix bed, with resulting problems of plugging and channeling [4]. Hence, modifying the AF process is required to minimize and overcome existing deficiencies faced by both UASB and AF reactors. Use of internal packing as an alternative to retain biomass in the UASB reactor is a suitable solution for the aforementioned problem. The packing medium in the UASB reactor is intended to increase retention of solids by dampening short circuiting, improving gas/liquid/solid separation, and providing surface for biomass attachment. A reactor of this kind is referred to as ahybrid Upflow Anaerobic Sludge-Filter Bed (UASFB) reactor in this study. This kind of reactor hybridizes the advantages of both UASB and Upflow Anaerobic Filter (UAF) processes, while minimizing their limitations [6], Using packing media only in the top portion of the reactor minimizes channeling problems associated to UAF and loss of biomass due to flotation associated to poorly performing UASB reactors.
In UASB reactors, the different zones are idealized with different flow patterns. Sludge blankets and sludge beds are mostly described by a CSTR flow pattern with bypass. The clarifier zone is described by the axially dispersed plug flow model. The application of these models for actual full-scale reactors is yet to be studied because these models do not consider the existence of non-ideal conditions in full-scale reactors. These non-ideal conditions may include nonexistence of different zones, improper flow distribution, and dead zones. Reactor models for UASB reactors consider many questionable assumptions like spherical granules, steady-state operation, description of substrate degradation by simple Monod kinetics, no substrate/product inhibition, and pH effect. Further studies are required to develop models that do not depend too much on these assumptions for their development.
In developing kinetic models for both UASBs and AFBRs, granule structure plays an important role. Studies show that the structure of the granules and bacterial composition depends on the type of effluent being treated. Various theories are provided to support the layered and un- layered structures of the granules. The variation of granule structure within the same reactor remains currently unexplained. Incorporating this variation into the models poses a challenge for modelers.
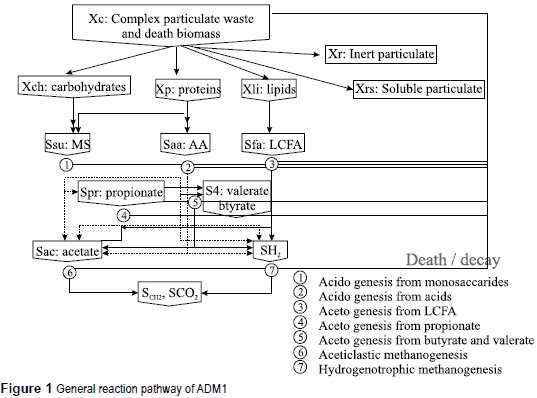
Relatively recently, the International Water Association (IWA) task group for mathematical modeling of anaerobic digestion processes developed a common model that can be used by researches and practitioners (Anaerobic Digestion Model, ADM1, [7]). This model has a structure similar to that of the IWA Activated Sludge Models (ASM) that has been accepted by practitioners over the last 20 years. The ADM1 model is described in considerable detail in the STR No 13, a report prepared by the IWA task group for mathematical modeling of anaerobic digestion processes. The following provides a brief overview of the model only for the purposes of this work.The ADM1 model is a structured model that reflects the major processes involved in the conversion of complex organic substrates into CH4 and C02 and inert byproducts. In figure 1, an overview of the substrates and conversion processes that are addressed by the model is presented.
Extracellular solubilization steps are divided into disintegration and hydrolysis of which the first is largely a non-biological step and converts complex solids into inert substances, carbohydrates, proteins, and lipids. The second step is enzymatic hydrolysis of particulate carbohydrates, proteins, and lipids to monosaccharides, amino acids and Long-Chain Fatty Acids (LCFA), respectively. Disintegration is mainly included to describe degradation of composite particulate material with lumped characteristics (such as waste activated sludge (WAS)), while the hydrolysis step is to describe well-defined relatively pure substrates (such as cellulose, starch and protein feeds). Monosaccharides and amino acids are fermented to produce Volatile Fatty Acids (VFAs - acidogenesis) and H2. LCFA are oxidized anaerobically to produce acetate and H2. Propionate, butyrate, and valerate are converted to acetate (acetogenesis) and H2. CH4 is produced by both cleavage of acetate to CH4 (aceticlastic methanogenesis) and reduction of CO2 by H2 to produce CH4 (hydrogenotrophic methanogenesis).
To address these mechanisms, the model employs 26 state variables to describe the behavior of soluble and particulate components. All organic species and molecular hydrogen are described in terms of Chemical Oxygen Demand (COD). Nitrogenous species and inorganic carbon species are described in terms of their molar concentrations. Soluble components (represented with a capital ''''S'') are those that can pass through microbial cellular walls and include the monomers of complex polymers (sugars, amino acids, LCFAs), volatile fatty acids (propionate, butyrate, valerate, acetate), hydrogen and methane.
Particulate species consist of either active biomass species or particulate substances incapable of directly passing through bacterial cell walls. In figure 1, particulate species are those with a capital ''X''. The microbial species considered in the model include sugar fermenters (Xsu), amino acid fermenters (Xaa), LCFA oxidizers (Xfa), butyrate and valerate oxidizers (Xc4), propionate oxidizers (Xpro), aceticlastic methanogens (Xac), and hydrogenotrophic methanogens (Xh2). Non-microbial particulate species include complex organics that either enter the process in the influent or result from the death and decay of microbial species and products of disintegration of complex organics. This latter group consists of carbohydrates, proteins, and LCFAs.
Substrate conversion processes are described by a number of kinetic expressions that describe conversion rates in terms of substrate concentrations and rate constants. Disintegration of Xc and hydrolysis of Xch, Xpr, and Xli are described by first-order rate expressions. Substrate-based uptake Monod-type is used as the basis for all intracellular biochemical reactions. Death of biomass is represented by first-order kinetics and dead biomass is maintained in the system as composite particulate material.
A number of the conversion processes active in anaerobic digestion of municipal sludge can be inhibited by accumulation of intermediate products like H2;. ammonia, or by extreme pH. In the model, inhibition functions include pH (all groups), hydrogen (acetogenic groups), and free ammonia (aceticlastic methanogens). Inhibition caused by H2 and free ammonia is implemented in the model by employing rate multipliers that reflect non–competitive inhibition. An empirical correlation is employed as a process rate multiplier to reflect the effects of extreme pH. Liquid-gas mass transfer of gaseous components (CH4, CO2 and H2) is described by mass-transfer relationships. Hence, the application of the model's equations requires separate mass balances for the liquid and gas phases of the components.
The ADM1 does not describe all the mechanisms occurring in anaerobic digestion (such as precipitation of solids and sulfate reduction, for example). However, the aim is a tool that allows sufficiently accurate predictions to be useful in process development, operation, and optimization. Because of varying demands in these fields, a different degree of model calibration and validation will be required in each case, as will be shown in the first section of this study with the biodégradation of wine distillery vinasses in a hybrid UASFB reactor. In the second section, the objective will be to better characterize the disintegration and hydrolysis steps and to integrate them into ADM1 to obtain a model able to predict and interpret results from thermophilic anaerobic digestion of thermally pretreated WAS.
Waste activated sludge is mainly composed of proteins and, thus, can release a large amount of ammonia during anaerobic degradation [8]. Although ammonia is an important buffer and an essential nutrient for anaerobic microbes, high ammonia concentrations can decrease microbial activities, particularly methanogens [9], Pretreatment technologies are often a way to optimize WAS conversion into methane. Over the years, several pretreatments were implemented and studied in the literature: physical [10], chemical [11], biological [12], and thermal [8] treatment to pre-hydrolyze the particulate organic matter and make it more available to the anaerobic biomass. In particular, thermal pretreatment can be combined with mesophilic anaerobic digestion and leads to increased biogas quantity and production rates [13], energy costs being covered by the additional biogas production [14]. Climent [15] and Bougrier [16] also underlined the positive impact of solubilisation of particulate organic matter on biogas production during anaerobic digestion. However, very few studies analyzed the combination of thermal pretreatment with thermophilic WAS anaerobic digestion, being Skiadas [17] and Jolis [18], practically the only papers available in the literature on this topic.
Materials and methods
A hybrid Up-flow Anaerobic Sludge Filter Bed (UASFB) reactor
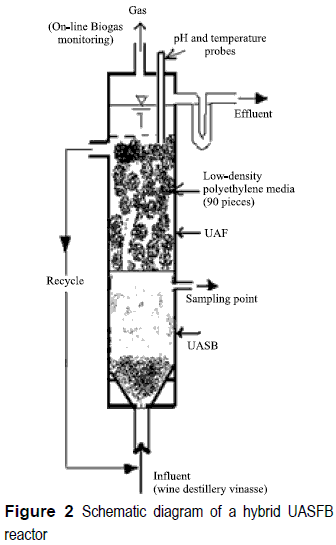
The schematic diagram of the laboratory scale UASFB reactor (diameter: 12 cm; height: 117 cm; effective volume: 9.8 l) used in this study is shown in figure 2. The reactor column was made up ofPlexiglas and comprised two compartments: the bottom part was operated as a UASB reactor, whereas the top part was operated as an anaerobic filter. The top portion of the UASFB reactor was randomly packed with 90 pieces of small cylindrical, buoyant polyethylene packing media (height: 29 mm; diameter: 30-35 mm; density: 0.93 kg/m3), and baffled with 16 partitions. Fifty percent of the reactor volume (excluding the 30- cm high headspace) was filled with the packing media.
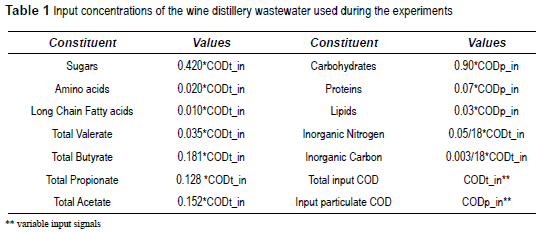
The reactor was equipped with a continuous internal recirculation system from top to bottom (recirculation rate: 9 1/h). Recirculation was done mainly to eliminate the possibility of high organic loading close to the feed port and to favor better wastewater/sludge contact. The digester was seeded with granules (15% by total volume) originating from a UASB digester treating cheese wastewaters. This hybrid UASFB reactor was operated for a total period of 232 days at 33 °C. Continuous feeding of the reactor was started with an initial Organic Load Rate (OLR) of 3.1 g COD/l d. Organic load rate was then increased stepwise by increasing the substrate concentration from 3.1 to 21.7 g/1 (around 95% of the total COD was soluble), while maintaining a constant HRT of 1.15 days. An 80% COD removal efficiency was considered the threshold level in this study to operate the UASFB reactor. The OLR was progressively increased by 20-30% once or twice a week until COD removal dropped below 80%. The feed was supplemented with nutrients to obtain a COD: N: P ratio of 400:7:1 in the wastewater. Feed pH was adjusted to 6- 6.5 by using a 6 N sodium hydroxide. Performance of the UASFB reactor was evaluated by monitoring total (CODt) and soluble (CODs) chemical oxygen demand, suspended solids (SS), volatile suspended solids (VSS), and alkalinity according to the Standard Methods for Examination of Water and Wastewater, [19] at reactor inlet and outlet. VFAs were determined by using gas chromatography (GC- 8000 Fisions instrument) equipped with a flame ionization detector with an automatic sampler AS 800. Biogas production was measured online. The percentages of methane (CH4) and carbon dioxide (CO2) in the biogas were determined by using a gas chromatograph (Shimadzu GC-8A), with argon as the carrier gas, equipped with a thermal conductivity detector and connected to an integrator (Shimadzu C-R8A). Experiments were performed with distillery vinasse (wine residue after distillation), obtained from a local distillery around Narbonne, France. In this type of wastewater, soluble COD is mainly present as monosaccharides (Ssu in ADM1) and little as amino acids (Saa) and long-chain fatty acids (Sfa). Particulate COD is mainly present in the form of carbohydrates (Xch), in addition to some composites (Xc), proteins (Xpr), and lipids (Xli). The input VFA values were calculated from measured concentrations of acetate (Sac), propionate (Spro), butyrate (Sbu), and valerate (Sva).
The initial pH resulted from the ionized forms of VFAs, bicarbonate, ammonia, and cation/ anion concentrations. Ammonia (SIN) and bicarbonate (SIC) were measured by Keljdahl's method and using a TOC meter, respectively.
Anion concentration (San) was taken equal to SIN and cation concentration (Scat) was adjusted in each case, according to the initial experimental pH. The concentrations of these individual components, used in the model as process inputs, are shown in table 1.
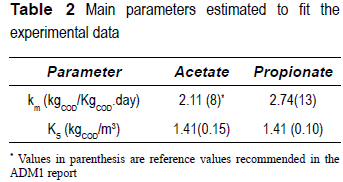
Previous experience in simulating the behavior of a reactor fed with the same wine distillery wastewater [20] led to identifying the main ADM1 parameters, which need to be modified to reasonably reflect the experimental data. Only the maximum specific-substrate uptake rate (km) and the half-saturation constant (Ks) for acetate and propionate were calibrated to fit the data (table 2). The difference between hydraulic and solid retention times (i.e., HRT and SRT) because of the biofilm present in the reactor was modeled by adding an extra term, that is, residence time of solids (tres,X) in the biomass equation, as recommended in the ADM1 report [7],
Batch thermophilic anaerobic digestion of thermally pretreated l/MAS
Waste Activated Sludge samples from a highly loaded wastewater treatment plant in France were used during the experiments. Thermal hydrolysis was carried out in a 10 L agitated autoclave (Autoclave, class IV), allowing temperature increase by electricity. Three temperature treatments were chosen: 110 C, 165 C, and 220 C. Once the desired temperature was reached, treatments lasted for 30 min. Chemical Oxygen Demand (COD) solubilization was used to evaluate the impact of thermal pretreatment and was expressed as a percentage, according to the equation (1).
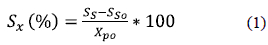
Where SS and SSO are the concentrations measured in the soluble fraction of treated and untreated sludge, respectively, and Xp0 is the concentration measured in the particulate fraction of untreated sludge. Biochemical Methane Potential (BMP) was based on measurements of the end product (biogas) and intermediate products like VFAs in a closed reactor wherein a measured quantity of substrate was introduced with a measured quantity of inoculum. The experiment was performed under favorable conditions for anaerobic digestion of sludge. Five reactors, with a 3.5 L volume each, were used in parallel. Anaerobic batch reactors were kept at 55°C (thermophilic conditions) by water circulation in a water jacket. The inoculum was taken from a full-scale sludge anaerobic digester. One reactor was used with no feed to quantify the endogenous activity of the inoculum. Other reactors were fed with untreated sludge and with sludge treated at 110 °C, 165 C, and 220 C. Organic loading was 0.5 gC0D of WAS per gVS of inoculum. For each condition, four successive 22-day batch experiments were carried out to minimize the effect of the inoculum. At the beginning of each BMP test, the reactors were purged with an N2/C02 (75/25) gas mixture. Biogas production and pH were continuously measured. An electronic volumetric gas counter was used to monitor biogas production. During anaerobic digestion, total and soluble COD, VFAs, and biogas composition were monitored daily to follow the formation of by-products involved in biological reactions. In each case, only the fourth batch experiment was used to calibrate and validate the model to minimize the influence of the initial inoculum composition.
The soluble and particulate fractions were separated by centrifugation at 50,000 g, 15 min, and 5 °C, then by filtration through a cellulose acetate membrane with 0.45-mm pore size. Substrate characterization was conducted on the sludge samples to determine initial values of the model variables. Some measurements were performed on total and soluble fractions: COD, proteins (measured according to the Lowry method, [21]) and total sugars (measured with the Anthrone reduction method, [22]). Ammonia nitrogen, inorganic carbon, and VFAs were measured only in the soluble fraction. Lipids were measured according to the Soxhlet method by using petroleum ether as solvent, on both total and particulate fractions.
Results and discussion
A hybrid Up-flow Anaerobic Siudge Filter Bed (UASFB) reactor
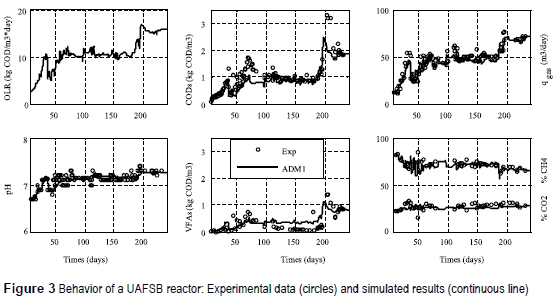
Figure 3 shows the experimental data for the entire study period, together with the varying input OLR and the simulated results from ADM1. As noted from figure 3, the model can simulate the dynamic evolutions of the main variables in the liquid and gas phases. After day 100, the model over-predicted VFA concentrations (mainly acetate). Apparently, the simulated rate at which acetate was converted into methane under the load imposed was somewhat under–estimated. This may have resulted from either under-estimation of the substrate consumption coefficients for aceticlastic methanogenesis or an over-estimation of the inhibition of this activity by ammonia [23], The model predicts well the dynamics of the biogas production rate and composition as a response of the load imposed. Small deviations in predicting biogas production and quality have been found. The differences can be explained with the non-optimization of several parameters; for instance, application of identical and non-optimized gas transfer coefficients. In fact, gas transfer coefficients may actually differ and the dependence on the specific reactor configuration applied has been neglected. The pH was also quite accurately simulated and the model was able to reflect the trends observed in experimental data. The pH prediction is closely related to the cation and anion concentrations in the reactor and the difference between the two concentrations. Because ion concentrations were not measured, these were then calculated by using the pH value and taking into account ammonia concentration, alkalinity, and VFA concentration in the reactor.
Batch thermophilic anaerobic digestion of thermally pretreated l WAS
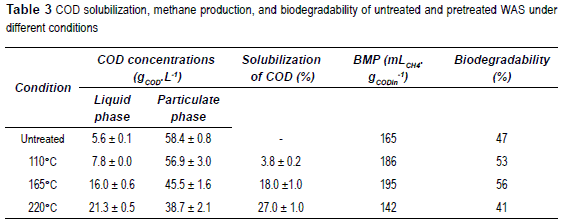
The parameter of COD solubilisation was used to evaluate the impact of thermal pretreatment on the transfer of organic matter from particulate phase to soluble phase obtained for the untreated and pretreated sludge. Results are summarized in table 3.
As already shown [15], the values of COD solubilisation increase with temperature from 3.8% (at 110 °C) to 27% (at 220 °C). Throughout applied thermal pretreatments, the total COD balance was maintained before and after treatment. The thermal pretreatment indeed leads to a transfer of particulate organic matter into the soluble phase (i.e., particulates lower than 0.45 μm) and can be assimilated to thermal hydrolysis. Thus, the application of thermal pretreatment to a largely particulate raw sludge (86%VS in our case) makes organic components more available to the anaerobic microorganisms and induces increased degradation rates and biogas volume produced.
Moreover, a maximum value can be noticed in methane production and WAS biodegradability when temperature reaches 165 °C, which can be considered the optimal pretreatment temperature. This finding agrees with the literature and earlier studies showed that a pretreatment temperature of 170 °C seems to be a limit for improvement of methane production [24]. At 220 °C, although a large solubilisation of particulate organic matter occurs, sludge biodegradability is in fact lower than the raw sludge biodegradability with only 142 ml CH4.gCODin-1 being produced.
Hydrolysis of particulate organic material has been traditionally modeled according to first–order kinetics and is usually considered as the rate- limiting step in anaerobic digestion [28]; some authors (Bryers [25] and Mata-Alvarez [26]) have pointed out that the mechanisms, stoichiometry, kinetics, and modeling of biological particulate hydrolysis have not yet been adequately studied. The complex multi-step process of carbohydrate, protein, and lipid hydrolysis may include multiple enzyme production, diffusion, adsorption, reaction, and enzyme deactivation steps [27]. Consequently, the first-order kinetics appears not applicable under all circumstances and in-depth understanding of the different processes involved is needed to accurately describe the disintegration and hydrolysis steps.
Furthermore, it has been shown that models in which disintegration/hydrolysis is coupled to the growth of disintegration/hydrolytic bacteria and to substrate heterogeneity work well even at high or fluctuant organic loading. In particular, the Contois model has been demonstrated to be well adapted to represent different experimental data sets from a wide range of organic wastes [27], In addition, anaerobic biodegradation of WAS produces a large quantity of ammonia, which is the main cause of inhibition given that, as already pointed out, it is freely cell-membrane permeable. Inhibition is usually indicated by a decrease of the methane production and an accumulation of VFAs, [28]. Free ammonia inhibition is included in ADM1 for aceticlastic methanogens using non-competitive functions; however, in our experiments of pretreated WAS, acetate was not completely degraded and, in some cases, a second phase of acetate production was observed. This phenomenon cannot be explained with the non-competitive function included in ADM1 for modeling free ammonia inhibition of aceticlastic methanogens and the following Hill function [29] was used instead:
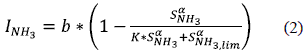
INH3 is free ammonia inhibition factor of aceticlastic methanogens,
b is the maximum desired value for ammonia inhibition,
SNH3 is the free ammonia concentration (kmoleN.m-3),
SNH3, lim is the mean free ammonia threshold concentration (kmoleN.m-3),
K is a tuning parameter, and
α is the Hill coefficient that defines the slope of the drop in the inhibition function.
These additional process rates and stoichiometry of the modified ADM1 were included in the ADM1 model. The remaining reactions (i.e., acidogenesis, acetogenesis, and methanogenesis) are strictly equivalent to those present in the standard ADM1.
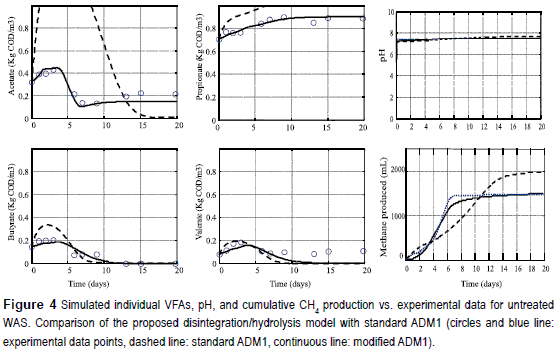
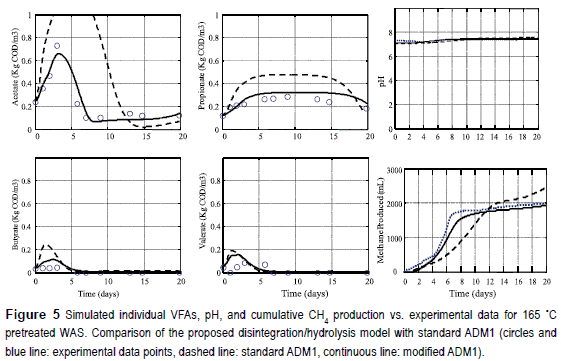
To summarize, this modified ADM1 involves a few additional parameters: three disintegration biochemical parameters of composites Xc (i.e., km,Xc KS,Xc, and kdec,Xc), nine hydrolysis biochemical process parameters for carbohydrates, proteins, and lipids (i.e., km,ch, KS,ch, kdec,ch, km,pr, kS,pr, kdec,p, km,li, KS,li and kdec,li, respectively) and four stoichiometric parameters (YXc, Ych, Ypr, and Yli). Their tuning was performed by using the experimental data set obtained from the batch reactor fed with untreated WAS, while the other three experimental data sets (i.e., those obtained from batch reactors fed with WAS thermally pretreated at 110, 165, and 220,°C) were used to validate the values obtained {i.e., the modified ADM1 was then simulated by using the model parameter values determined from the untreated WAS experiment).
The modified ADM 1 was implemented by using MatLab/Simulink. Values for initial conditions of most of the model variables were directly obtained from the experimental measurements on the WAS samples. The behavior of the modified anaerobic digestion model was compared to that of the standard ADM1 and to experimental results in simulating the behavior of a batch thermophilic anaerobic digestion of thermally pretreated WAS.
Figures 4 and 5 display the simulated (with both models) and experimental results for the untreated and 165 °C pretreated WAS. Similar results were obtained for the other two pretreated WASs, but they are not shown due to space limitations. As can be seen, model simulations closely follow the dynamic evolutions of the main variables in the liquid and gas phases. The model predicts well the dynamics of the biogas production rate as a response of the pretreatment imposed. Small deviations in predicting the cumulative biogas production were found. The pH model simulation reflected the trends observed in experimental data. For both untreated and pretreated WAS, pH was in general within the range of 7.18 - 7.59, with the low values corresponding to periods where VFAs accumulate in the thermophilic batch reactors. In all cases, pH varies within 0.3 units, even when the process was inhibited and VFA accumulated. The relatively large resistance to pH changes was probably due to reactor buffering capacity.
Conclusions
First, the ADM1 model was able to closely simulate the dynamic evolutions of the main variables in the liquid and gas phases in the anaerobic digestion of wine distillery wastewater in a hybrid UASFB reactor, when only the Kinetic parameters of acetate and propionate were modified.
Second, a slightly modified IWA ADM1 model for thermophilic anaerobic digestion of thermally pretreated Waste Activated Sludge was implemented by using batch experimental data sets. The model was based on the following hypothesis: (a) disintegration and hydrolysis processes are described according to the Contois model and (b) ammonia inhibition for aceticlastic methanogens can be represented according to the general Hill function. Predictions by the model, using the parameters established in this study, agreed well with the data measured under different pretreatment conditions. The resulting model was explained the dynamics of acetate accumulation obtained in some batch experiments and possibly characterized by two peaks of acetate concentration, the result of different hydrolysis rates for fats and proteins.
References
1. K. Boe. On-line monitoring and control of the biogas process. Thesis. Technical University of Denmark. Lyngby, Denmark. 2006. pp. 12-28. [ Links ]
2. J. Mata, P. Llabres. ''Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives''. Bioresour. Technol. Vol. 74. 2000. pp. 3-16. [ Links ]
3. V. Saravanan. T. Sreekrishnan. ''Modeling anaerobic biofilm reactors – A review''. Journal of Environmental Management. Vol. 81. 2006. pp. 1-18. [ Links ]
4. J. Jhung, E. Choi. ''A comparative study of UASB and anaerobic fixed film reactors with development of sludge granulation''. Water Res. Vol. 29. 1995. pp.271–277. [ Links ]
5. J. Reynolds, E. Colleran. Comparison of start-up and operation of anaerobic fixed bed and hybrid sludge bed reactors treating whey wastewater. Proceedings of the Preprints EWPCA conference on Anaerobic treatment a Grown-up Technology. Amsterdam. 1986. pp. 515-531. [ Links ]
6. K. Lo, P. Liao, Y. Gao. ''Anaerobic treatment of swine wastewater using hybrid UASB reactors''. Bioresour. Technol. Vol. 47. 1994. pp. 153-157. [ Links ]
7. D. Batstone, J. Keller, I. Angelidaki, S. Kalyuzhnyi, S. Pavlostathis, A. Rozzi, W. Sanders, H. Siegrist, V. Vavilin. Anaerobic Digestion Model No 1. Scientific and technical report 13. International Water Association (IWA). London, UK. 2002. pp. 4-40. [ Links ]
8. T. Jeong, G. Cha, S. Choi, C. Jeon. ''Evaluation of methane production by the thermal pretreatment of waste activated sludge in an anaerobic digester''. Journal of Industrial and Engineering Chemistry. Vol. 13. 2007. pp. 558-563. [ Links ]
9. I. Angelidaki, B. Ahring. ''Anaerobic thermophilic digestion of manure at different ammonia loads: effect of temperature''. Water Research. Vol. 28. 1994. pp. 727-731. [ Links ]
10. K. Nickel, U. Neis. ''Ultrasonic disintegration of biosolids for improved biodegradation''. Ultrasonics Sonochemistry. Vol. 14. 2007. pp. 450-455. [ Links ]
11. I. Ardic, F. Taner. ''Effects of thermal, chemical and thermochemical pretreatments to increase biogas production yield of chicken manure''. Fresenius Environmental Bulletin. Vol. 14. 2005. pp. 373-380. [ Links ]
12. A. Davidsson, J. Wawrzynczyk, O. Norrlow, J. Jansen. ''Strategies for enzyme dosing to enhance anaerobic digestion of sewage sludge''. Journal of Residuals Science & Technology. Vol. 4. 2007. pp. 1-7. [ Links ]
13. Y. Li, T. Noike. ''Upgrading of anaerobic digestion of waste activated sludge by thermal pretreatment''. Water Science and Technology. Vol. 26. 1992. pp. 857–866. [ Links ]
14. C. Bougrier, J. Delgenes, H. Carrere. ''Impacts of thermal pre-treatments on the semi-continuous anaerobic digestion of waste activated sludge''. Biochemical Engineering Journal. Vol. 34. 2007. pp. 20-27. [ Links ]
15. M. Climent, I. Ferrer, M. Baeza, A. Artola, F. Vazquez, X. Font. ''Effects of thermal and mechanical pretreatments of secondary sludge on biogas production under thermophilic conditions''. Chemical Engineering Journal. Vol. 133. 2007. pp. 335-342. [ Links ]
16. C. Bougrier, J. Delgenes, H. Carrere. ''Effects of thermal treatments on five different waste activated sludge samples solubilisation, physical properties and anaerobic digestion''. Chemical Engineering Journal. Vol. 139. 2008. pp. 638-649. [ Links ]
17. I. Skiadas, H. Gavala, J. Lu, B. Ahring. ''Thermal pretreatment of primary and secondary sludge at 70 degrees C prior to anaerobic digestion''. Water Science and Technology. Vol. 52. 2005. pp. 161-166. [ Links ]
18. D, Jolis. ''High-solids anaerobic digestion of municipal sludge pretreated by thermal hydrolysis''. Water Environment Research. Vol. 80. 2008. pp. 654-662. [ Links ]
19. American Public Health Association (APHA), American Water Works Association, Water Pollution Control Federation. Standard Methods for the Examination of Water and Wastewater. 18th ed. Washington DC, USA. 1992. pp. 380-425. [ Links ]
20. I. Ramirez, J. Steyer. ''Modeling microbial diversity in anaerobic digestion''. Water Sci. Technol. Vol. 57. 2008. pp. 265-270. [ Links ]
21. O. Lowry, N. Rosebrough, A. Fau, R. Randall. ''Protein measurement with the Folin reagent''. Journal of Biological Chemistry. Vol. 193. 1951. pp. 265-275. [ Links ]
22. R. Drey wood. ''Qualitative test for carbohydrate material''. Industrial and Engineering Chemistry Analytical Edition. Vol. 18. 1946. pp. 499-504. [ Links ]
23. W. Parker. ''Application of the ADM1 model to advanced anaerobic digestion''. Bioresour. Technol. Vol. 96. 2005. pp. 1832-1842. [ Links ]
24. S. Pavlostathis, E. Giraldo. ''Kinetics of anaerobic treatment: a critical reiew''. Critical Reviews in Environmental Control. Vol. 21. 1991. pp. 411-490. [ Links ]
25. J. Bryers. ''Structures modeling of the anaerobic digestion of biomass particulate''. Biotechnology and Bioengineering. Vol. 27. 1985. pp. 638-649. [ Links ]
26. J. Mata. ''A simulation study of a continuous two phase dry digestion system''. Biotechnology and Bioengineering. Vol. 34. 1989. pp. 609-616. [ Links ]
27. V. vavilin, B. Fernandez, J. Palatsi, X. Flotats. ''Hydrolysis kinetics in anaerobic degradation of particulate organic material: an overview''. Waste Management. Vol. 28. 2008. pp. 941-953. [ Links ]
28. Y. Chen, S. Jiang, H. Yuan, Q. Zhou, G. Gu. ''Hydrolysis and acidification of waste activated sludge at different pHs''. Water Research. Vol. 41. 2008. pp. 683-689. [ Links ]
29. D. Hill, C. Barth, C. ''A dynamic model for simulation of animal waste digestion''. Journal of Water Pollution Control Federation. Vol. 49. 1977. pp. 2129-2143. [ Links ]