Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230
Rev.fac.ing.univ. Antioquia no.66 Medellín Jan./Mar. 2013
ARTÍCULO ORIGINAL
Hydrogeochemistry and pollution effects of an aquifer in Quaternary loess like sediments in the landfilling area of Mar del Plata, Argentina
Hidrogeoquímica y efectos de la contaminación en un acuífero en sedimentos loéssicos Cuaternarios en el área de rellenos sanitarios de Mar del Plata, Argentina
Daniel E. Martínez*1,2, Margarita Osterrieth 1,2
1CONICET - Instituto de Investigaciones Marinas y Costeras. U.N. de Mar del Plata. Casilla de Correo 722 (7600), Mar del Plata, Argentina.
2Instituto de Geología de Costas y del Cuaternario, U.N. de Mar del Plata. Casilla de Correo 722 (7600), Mar del Plata, Argentina..
*Autor de correspondencia: teléfono: + 54 + 233 4754060, fax: + 54 + 223 4753150, correo electrónico: demarti@mdp.edu.ar (D. Martínez)
(Recibido el 3 de noviembre de 2012. Aceptado el 18 de enero de 2013)
Abstract
Loess like sediments cover an area of about 1.800.000 km2 in the Pampa plain of Argentina, forming an aquifer system which is used for water supply for most of the cities a rural population in the region. This aquifer supplies water to agricultural productive activities that contribute to about a 60% of the national gross produce of the country. In this paper, detailed studies in a local sector of the aquifer near to Mar del Plata, in the landfilling area of the city, are performed. The main goal is to characterise the hydrogeochemical processes giving the chemical groundwater composition, and to analyze the impact of the infiltration of the leachate from a neighbouring landfill on some samples. Five wells were drilled to take sediments and water samples. The textural and mineralogical composition of the aquifer sediments was analysed and the chemical composition of groundwater was determined. The equilibrium relationship between the solid phase and groundwater was considered using specific computer codes. The achieved conclusions were that the chemical composition of groundwater is mainly due to calcite equilibrium and cationic exchange with calcium uptake and sodium release. The dissolution of amorphous silica minerals, and subordinate silicate hydrolysis, are responsible of the characteristic high dissolved silica concentrations. High chloride and nitrate contents result from leachate infiltration, but the main geochemical processes in the mix are the same.
Keywords: Hydrochemistry, Loess sediments, Argentina, mineral saturation, silica contents
Resumen
Sedimentos de tipo loéssico cubren una superficie aproximada de 1.800.000 km2 en la llanura Pampeana de Argentina, formando un sistema acuífero que es utilizado para abastecimiento de agua a ciudades y la población rural en la región. Este sistema acuífero suministra el agua que sustenta las actividades productivas que contribuyen en un 60% al producto bruto nacional. Es este trabajo se realiza un estudio detallado en un sector del acuífero próximo a la ciudad de Mar del Plata, provincia de Buenos Aires, en el que se localizan tres rellenos sanitarios. El principal objetivo es caracterizar los procesos geoquímicos que dan origen a la composición observada del agua subterránea. Se perforaron cinco pozos para la toma de muestras de agua y sedimentos, y evaluar el impacto de la infiltración del lixiviado sobre esos procesos. Se determinó la composición textural y mineralógica de los sedimentos y la composición química del agua. Las relaciones de equilibrio entre la fase sólida y la solución se determinó utilizando programas específicos. Se concluye que la composición química observada en la aguas es consecuencia principalmente de procesos de equilibrio con calcita e intercambio catiónico con adsorción de calcio y liberación de sodio. La disolución de minerales de sílice amorfo, y la hidrólisis de silicatos en forma subordinada, da origen a altas concentraciones de sílice en las aguas. La mezcla con la infiltración del lixiviado incrementa las concentraciones de los iones conservativos cloruro y nitrato, pero no se modifican en general los procesos hidrogeoquímicos mayoritarios.
Palabras clave: Hidrogeoquímica, sedimentos loéssicos, Argentina, saturación de minerales, contenido de sílice
Introduction
The so-called Pampean sediments [1] represent one of the largest geological formations in Argentina, covering approximately 1,800,000 km2 (figure 1). These sediments, of silt and silty-sand composition, with interstratified silt-clay layers, are of eolic and fluvial-eolic origin, dating back to the middle to Upper Quaternary. They contain a lot of aquifers which, as a whole, conform the aquifer system that provides one of the most densely populated and most economically active with a supply of fresh water. In fact, this area accounts for more than a 60% of the national gross product of the country [2]. The quality of the water obtained varies greatly along this system of aquifers, with some areas in the Pampean region evidencing problems related to high contents of arsenic and fluoride [3 - 9] or nitrate [10].
In order to analyze a hydrogeological system like this, it is necessary to look into the interrelation among its three phases: a gas phase resulting from an exchange with the atmosphere or from biogeochemical processes in the unsaturated zone, a solid phase formed by the minerals that constitute the porous media, and a liquid phase, the aqueous solution that is groundwater. Chemical equilibrium as a way of understanding has been early developed in the work of Chebotarev [11], but especially the work of Garrels and Christ [12] highlighted the usefulness of this tool, leading it to be the most applied methods of analysis at present.
The main goal of this work is to increase the knowledge of the hydrogeochemistry of the aquifers formed by Pampean sediments by mean of a detailed study of the mineralogical composition of the sediment matrix in contact with the analyzed groundwater samples and the complementary measurement of silica and aluminum in the solution, which allows the consideration of the equilibrium with silicate minerals. Some specific features of the restricted considered area are very locals, i.e. coastal location, marine aerosol contribution in recharge, small thickness of the aquifer, proximity of the hydrogeological basement, etc., and especially the fact that the wells are located very near to a landfill which is operating since 1995, occupying a surface of 40 ha. Previous studies have characterized the groundwater pollution in the area [13 - 15] at a descriptive level.
Nevertheless few previous contributions relate hydrochemistry with a detailed mineralogy of the sediments also including clay cristallinity and composition, and which specific processes take place if high recharge is partially affected by landfills leachate. This paper is focused in a single area of the Pampean Plain, but the application of a whole study involving mineralogy, chemistry of sediments, hydrochemistry and processes modeling under chemical equilibrium conditions make it a contribution that rises regional meaning in reference with the study methodology.
The mineralogical composition of the loess-like sediments was described in the study area in an important previous work on the Pampean Loess by Teruggi [16] analyzing samples taken near to Mar del Plata and La Plata, and describing a constant composition in all the considered samples. The content of calcite in this loess and loess-like sediments is about 2% and 4% [17]. A study carried on Pampean sediments in the southeast o the province of Córdoba [3] assign the major composition of sand and silt fractions to feldspars (40-75%) followed by volcanic glass (25-50%), and decreasing proportions of quartz, calcite, muscovite and rock debris. Illite is the dominant species in the clay fraction. Volcanic glass abundance is a distinctive feature of argentine loess, differentiating it from the loess in China that having a similar composition and also CaCO3 content, doesn't include volcanic shards [18].
Study area characterization
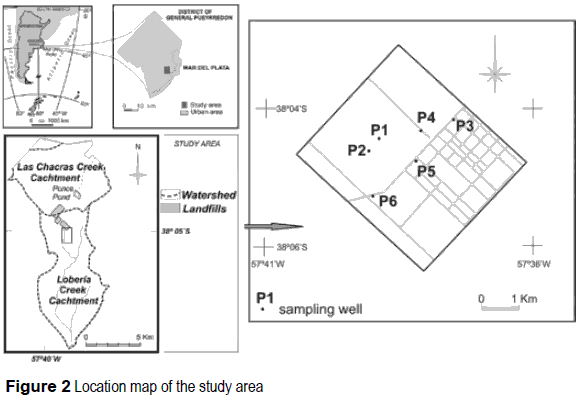
The study area is presented in figure 2, corresponding to the headwaters sector in the basin of the Loberia creek (total area 15 km2), placed in the District of General Pueyrredón, near to the city of Mar del Piata, at 38°05' lat. S and 57°38' long. W.
The climate is dry sub-humid mesothermal type "B2" [19]. Over the past 10 years, annual precipitation values have ranged from 703 to 1.400 mm/year, with an average of 943 mm/year.
The catchment of the Lobería Creek is characterised by a hilly relief, which is due to a shallow structural control. Blocks formed by orthoquartzites of Early Palaeozoic age corresponding to Balcarce Formation [20]. They are the southernmost spurs of the Tandilia Range [21], and a thickness of more than 400 m has been described in the area, overlying Precambrian metamorphic rocks. Three fault systems are recognized in the Paleozoic bedrock, with NW-SE, NE-SW, and E-W trends with which three joint systems are associated [21].
The orthoquartzites comprise the impermeable hydrogeological basement of the region [22]. They have been reached by the study wells at depth between 4 and 50 m below the sedimentary cover of loess-like sediments. Though generally considered impermeable, the joints of these orthoquarzites produce a secondary porosity, which considered negligible as an aquifer with regard to the overlying clastic aquifer. The most common sequence presents orthoquartzites topped by loess-like Pleistocene-Holocene sediments and very thin sandy sediments. Hydrologically, these loess-like deposits represent the principal aquifer in the region. The clastic thickness of the analyzed basin was determined by means of drilling and geoelectrical prospecting [13].
The basement reaches a depth of more than 100 m in the distal zone of the basin. The aquifer of Mar del Plata is a Pleistocene sedimentary sequence (silt and silty-sand). The permeability varies from 10-15 m/day. Pumping tests show values for transmissivity of 500 m2/day in the southwestern zone of the district, between 500 and 700 m2/day in the urban zone, and between 1,000 and 1,200 m2/day in the northern and northwestern rural zones. The storage coefficient is in the order of 0.001. The recharge takes place in the upper part of the aquifer in practically the entire exposed area. The recharge reaches the highest levels of the aquifer and from there drains into deeper levels. Rainfall infiltration produces the recharge to the systems. The annual average rainfall for the period 1901-1987 was 851.6 mm. The losses produced by evapotranspiration have been calculated by the Thornthwaite method in 719.2 mm/year, leaving a surplus of 132.4 mm/year.
The underground flow direction is the same as the surface water flow direction on the northern slope, i.e., it is NW-SE. The average hydraulic gradient in the foothills is 0.015, whereas on the plains it is 0.008 [13, 15].
Methods
Six boreholes of a 4" diameter were built using a rotary machine in a zone of low thickness of the sedimentary section (figure 2). The boreholes were drilled almost on the water divide, corresponding then to a recharge zone where groundwater starts the flow sense towards the SE. The final depth of each well was: P1: 12 m; P2: 6m; P3:11.5 m; P4:15 m; P5: 39.5 m; P6: 12 m, reaching the quartzitic bedrock the wells P1, P2, P3 and P5. A PVC casing was used in each of them. Debris samples were taken each meter of depth to be analyzed in laboratory.
Water samples were taken in each well, including an additional sampling of wells P4 and P5 using the pump at different depths. Water table depth was recorded by using a bipolar probe, and the values were between 2 m and 5 m. Physical-chemical parameters temperature, pH, electrical conductivity (EC) and alkalinity where measured "in situ" during sampling procedure. Partial pressure of CO2 (PCO2) is obtained if pH and alkalinity are measured as they are related according the expression [23].
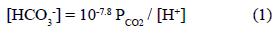
Codes like NETPATH [24] or PHREEQC [25] compute PCO2 automatically from the input alkalinity and pH data.
Considering the well logs, six representative sediment samples were selected to perform detailed studies. The samples correspond to different wells and different depths. The studies include textural analysis by sieving and pipette techniques and mineralogical description of the sand fraction using binocular magnifying glass, X-ray diffraction analysis of the mineralogy of clay fraction using a cooper radiation Philips instrument. Cationic exchange capacity (CEC) and organic matter content were also measured. The content of organic matter was determined using the chromic acid titration method [26]. The chemical composition of the selected sediment samples were analysed by acid digestion spectrometry ICP in order to give a more complete characterisation.
Water sampling methods were according to Kent and Payne [27] suggestions, especially in relation to pumping time to take representative samples. United Sates Geological Survey (USGS) techniques [28] were applied for water samples conservation. Standard techniques were used to measure the major ion concentrations. Analytical results were processed using PHREEQC2.0 code [25] in order to study equilibrium processes. Saturation indexes (SI) of mineral species were obtained, and some reaction paths were simulated. Netpath code [24] was used to perform mass balances to quantify the identified processes.
Results
Solid phase
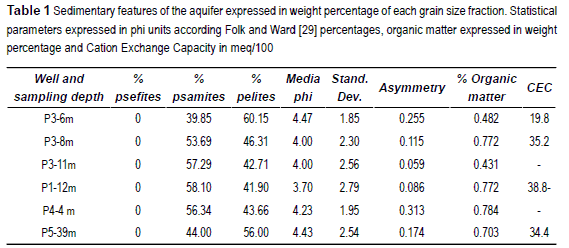
The textural analysis of the selected sediment samples shows that the material forming the Pampean aquifer present a normal distribution of is grain size, being comprise in the range between phi -0.5 and 11 (32 mm to 0.00005 mm). The modal fraction I in the range from 3.5 to 4.5 phi (0.088 mm to 0.044 mm), indicating that almost the 40% of the aquifer is formed by fine sand and coarse silt. The statistical parameters [29] of the analyzed samples are shown in table 1.
Quartz is the dominant mineral in the very fine sand fraction, followed by plagioclases, rock debris, potassium feldspar, volcanic glass, mica, pyroxene, amphibole, magnetite, calcite, amorphous silica (mainly silicophytolites and sponge needles), olivine and sillimanite. In general, the weathering degree of the minerals is inversely related to the order of abundance. The most weathering affected minerals are: silicophytolites, rock debris, olivine, pyroxene, mica, potassium feldspar, plagioclases, and finally quartz and volcanic glass that appears with a low weathering degree.
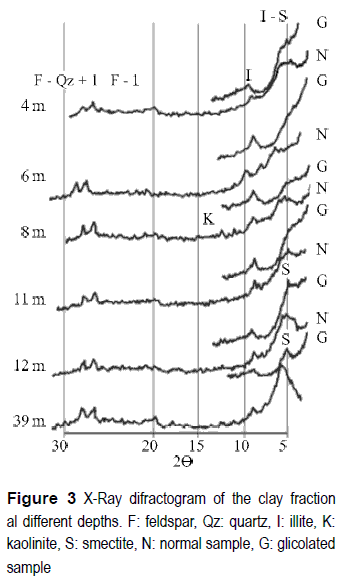
The mineralogy of the clay fraction (figure 3) shows a dominance of expansive minerals, of the type of the smectites to the deeper levels. In the upper levels the interstratified minerals are dominant. They are of the type illite-smectite with poorly defined reflections, only expressed in an expansive zone between 3° and 7° (2θ). Illite is found with the typical reflection at 1.004 nÅ in the natural and glicolated samples, and at 1.765 nÅ in the natural samples. This mineral increases its crystallographic definition to the deeper levels.
The definition of the reflection peaks of illite muscovite increases to the deeper levels, and more clear peaks of the reflection of calcium and magnesium smectites at 1.496 nÅ and calcium smectites in glicolated samples at 1.522/1.605 can be observed. Kaolinite is present as a trace in surface samples and increases lightly its abundance to deeper levels. Quartz, potassium feldspars and plagioclases are common in the clay fraction of all the studied samples.
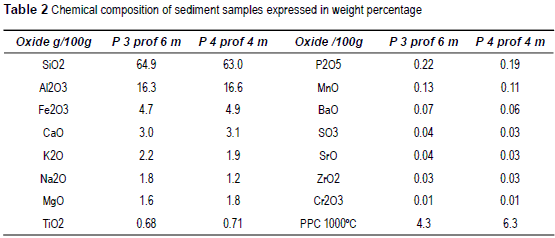
The chemical composition of two samples of sediments taken at 4 m and 6 m depth is shown in table 2.
Hydrogeochemistry
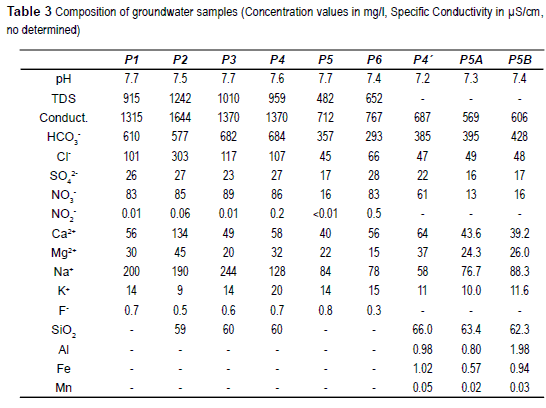
Reliability check of the analysis was done considering the electroneutrality balance. Most of the samples are below the 5% of difference, with the only exception of sample P4 which is about 16%. The results are used anyway considering that despite the analysis includes some non acceptable error; the main conclusions are not strongly affected because of that.
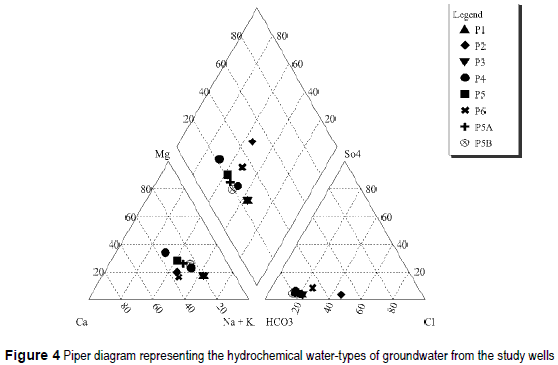
The water samples taken are bicarbonate waters, being sodium waters the samples from wells P1, P3, sodium being dominant cation the samples from wells P2, P4, P5, P5A, P5B, P6. Only the sample P4' presents HCO3-Ca/Mg composition. The hydrochemical water-types are represented in the Piper diagram of figure 4. The waters are low or medium salinity, with a salt content between 500 mg/L and 1.250 mg/L. The chemical composition of the samples and their pH and EC values are shown in table 3.
A main differentiation can be made in water samples. One group is integrated by samples P1, P2, P3 and P4 is characterized by EC higher than 1,300 μS/cm, chloride concentrations over 100 mg/l and nitrate concentrations exceeding 80 mg/l. On the other hand samples P4', P5, P5A and P5B are low salinity (EC < 750 μS/cm) and low chloride (< 70 mg/l) and nitrate (< 16 mg/l excepting P6 which is 61 mg/l) concentrations.
A preliminary explanation is that the first group, more saline and nitrate contaminated, correspond to the shallow boreholes which are closer to the landfill area. On the other side it is the well P5, which is farther from the landfill (about 500 m) and much deeper (39.5 m) because a higher aquifer thickness, and partially well P6 which is also farther. P1, P2, P3 and P4 are probably affected by a leachate infiltration enhanced by the low dilution in so slim saturated zone. P5, P5A and P5B samples, and sample P4' taken at the bottom of P4 seems not to be affected by leachate infiltration. Also P6 is far enough to the landfill to be affected by leachate infiltration, and its nitrate concentration can be attributed to farming practices in the surroundings.
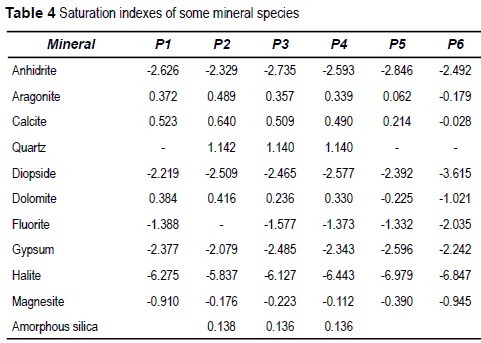
On the basis of the calculated concentrations as activities it is possible to obtain the saturation indexes (SI) of the solution respecting the minerals that can be formed with the measured components. The SI is
IS = log (Ionic Activity Product/Equilibrium constant)
Positive values of SI indicate over-saturated samples, and negative values indicate under- saturated samples. La IS values for some mineral species are shown in table 4.
Most of the samples are under-saturated in sulfate minerals or halogen salts, over-saturated in quartz, and near to equilibrium with carbonate minerals, being calcite the existing phase.
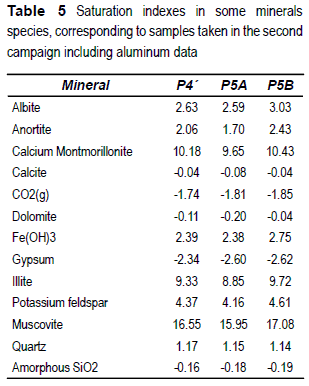
Aluminum was measured in the samples taken in the second sampling, allowing obtaining the SI of some aluminum-silicate minerals, as shown in table 5.
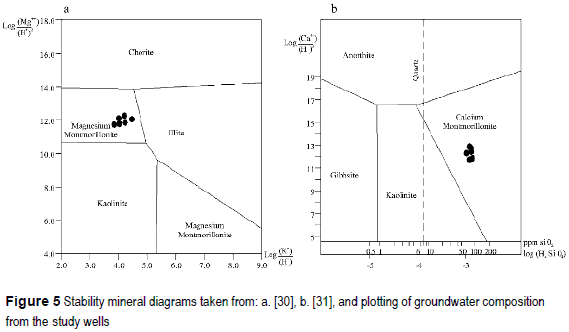
Using the activity ratios it is possible to plot the observed compositions in equilibrium diagrams as showed in figure 5.a. (taken from [30]). The ionic rations of the analyzed solutions are plotted in the field of the magnesium montmorillonite. Ionic ratios including calcium area shown in figure 5.b. (from [31]), where it is possible to see that the considered solutions are represented in the calcium-montmorillonite field. Using PHREEQC2.0 it is possible to simulate the reaction between anorthite and rainwater giving as product the neo-formation of calcium- montmorillonite. This reaction, forcing the previous calculated SI, gives as result that it is necessary to dissolve 2.53 μmol of anortite, resulting in the montmorillonite formation and the release of 3 μmol of Ca2+ and 5 μmol of Si.
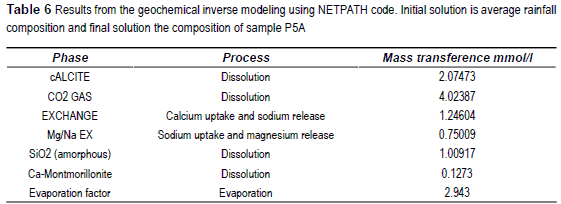
Taking into account the observed equilibrium, and inverse modeling using NETPTAH [24] was performed in order to quantify the processes that explain the observed compositions. One model was done to explain the non polluted composition model, and the initial solution used was the average composition of the rain water of Mar del Plata. This average composition is: pH 6.54, Na+ 18.33 ppm, K+ 0.2 ppm, Mg2+ 2.07 ppm, Ca2+ 3.52 ppm, Cl- 16.67 ppm, SO42+ 18.42 ppm and HCO3- 13.10 ppm. The final composition is that of sample P5A.
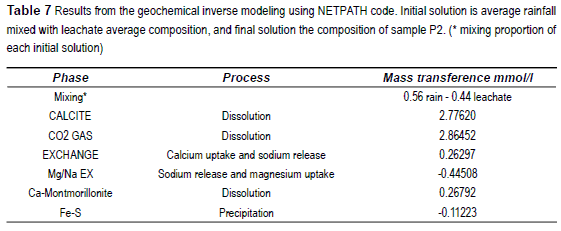
The models devoted to explain groundwater composition of the polluted wells use as initials a mix between rain water and leachate average composition [13] to achieve the final composition of sample P2. This average leachate composition is: pH 8.21, Na+ 426.5 ppm, K+ 22.8 ppm, Mg2+ 74.9 ppm, Ca2+ 77.4 ppm, Cl- 665.7 ppm, SO42- 62.3 ppm and HCO3- 587.2 ppm.
According the observed SI the considered processes were carbonate equilibrium, amorphous silica equilibrium, loss or dissolution of CO2 and cationic exchange. The solid phase calcium montmorillonite was also included. The increase in chloride concentration was used to calculate the rain water evaporation factor. The obtained results were very similar for the three final solutions, showing below the model corresponding to sample P5A in table 6.
On the other hand the results of modeling for polluted groundwater are shown in table 7, considering almost the same processes but considering rain water and leachate mixing during recharge after reacting with the solid phase. The formation of iron monosulfides (FeS) was included to explain the sulfur decrease in the mass balance.
Discussion
The chemical composition of groundwater is the result of the interaction with the minerals present in the flow path. The ratio Ca2+/Mg2+ determines that calcite is the most stable carbonate mineral. The SI values for calcite are consistent with this sentence, being near to 0. SI of dolomite also is near to equilibrium, but this mineral has not been identified in the sediments of the aquifer, and its precipitation is not possible according to its diagenetic origin as determined by Berner [32]. Other minerals of fast reaction, like gypsum, halite or mirabilte are under-saturated, and then they must not be presents in the sediment in the study area.
Calcite SI indicates equilibrium conditions in the samples showed in table 5 , but oversaturation in the samples included in table 4. These oversaturation conditions correspond probably to sampling errors, being possible that the pH measurements in surface were affected by the sample aeration due to the pumping by a centrifugal pump.
The SI of the silicate minerals forming the aquifer matrix indicates oversaturation in all the cases. According the magnesium/potassium ratio represented in stability diagrams, magnesium montmorillonite is the stable clay mineral. Taking this into account it is possible that montmorillonite is a neoformation product mineral, while illite should be of detritic origin. Taking into account the ratio of calcium and proton activities, and the activity of silica in solution, calcium montmorillonite apperas as the stable phase against anortite, which unstable in this environment. Then it is again considered that anorthite is of detritic origin and calcium montmorillonite due to neoformation. The increase in the proportion of smectite toward deeper levels, together with the increase in its degree of crystallinity, supports the hypothesis of the neoformation of these minerals. Moreover, the modeling performed with PHREEQC demonstrated numerically the coherence of the reaction of incongruent dissolution of anorthite, the montmorillonite neoformation and the addition to solution of calcium and silica. The incongruent dissolution of silicates produces an effect on groundwater that is the addition of cations and silica, and in a secondary way the bicarbonate formation [23].
The SI value of amorphous silica of 0.16 shows an equilibrium state. The high concentration of silica, which is a characteristic of the pampeano aquifer, can be explained by the dissolution of the amorphous silica minerals, and in a lower degree by the weathering of silicates. Among the amorphous silica minerals the more abundant are volcanic glass and silicophytolites. In spite of the alteration of volcanic glass has been observed in other areas ofthe Pampean Plain [3], in the studied sector the volcanic glass shards are observed in general with a low degree of weathering. The dissolution of biogenic silica minerals has been evidenced for their high degree of weathering, bringing silica to the solution. Taking into account the dominant water type existing in the area (NaHCO3) it should be important to mention that Marshall and Warakomski [33] demonstrated that the solubility of amorphous silica is higher in NaHCO3 solutions. The amorphous silica minerals present in the argentine loess are the reason of the high contents of dissolved silica. Groundwater in the loess form China, not containing important proportions of amorphous silica, has just about 10% to 20% [18] of the amounts of silica determinate in this study.
Moreover the incongruent dissolution of silicates and monmorillonite neoformation involves a reaction of the type:
Cation-aluminum silicate + CO2 + H2O <—> amorphous aluminum silicate + SiO2 + cation
The cation composition given to the solution by the silicate dissolution processes are later affected by exchange processes in adsorption surfaces. Cation exchange capacity (CEC) measurements in the sediments result in values in the order of 30-40 meq/100 g. The main exchange processes in this aquifer have been specifically studied [34], determining in the recharge areas like that considered in this work the dominance of calcium uptake and sodium release. The cation adsorption selectivity determined for normal flow conditions from recharge to discharge zones is Ca>Mg>Na>K and is responsible of the evolution from calcium waters to sodium waters.
Inverse hydrogeochemical modeling applied to non polluted (i.e. low chloride and low nitrate groundwater) indicates that, starting from rain water, to achieve the composition of the sample P5A, it is necessary: to dissolve 2.047 mol of calcite, 4.024 mol of CO2 and 1.009 mol of amorphous silica. In the cationic exchange process 0.49 mol of Na+ and 0.75 mol of Mg2+ are released and 1.246 mol of Ca2+ are uptake. A small quantity of calcium montmorillonite must be dissolved to explain the Al3+ in solution. Nevertheless, it should be noted that Al3+ was not measured in rain water, considering 0 its concentration.
Inverse modeling applied to a contaminated sample indicates approximately equal proportions of landfill leachate infiltrates mixed with precipitation recharge. This mixing contributes with most of the high salinity, i.e. dissolved chloride and sodium, while the other ions seem to be affected by the same processes than in normal evolution.
Conclusions
The hydrochemistry of the Pampeano aquifer system is the result of the interaction of recharge water, mainly rain water, with the mineral phases forming the aquifer. Taking into account the mineralogical composition there are two main kind of processes: chemical equilibrium between the solution and some minerals, specially calcite and amorphous silica, and cationic exchange. Calcite is a mineral widely distributed in Pampean sediments present forming compact layers or disseminated as small concretions, and equilibrium condition can be assumed according the rapid reversible dissolution reaction. Amorphous silica is present as volcanic glass shards or silica minerals formed by biological (vegetal) processes known as silicophytolites. As mentioned silicophytolites appears more weathered than volcanic glass in the study area. Amorphous silica minerals dissolution is responsible of one of the most noticeable features of groundwater from loess pampean aquifers, which the high dissolved silica contents, ranging usually in the order of 50-70 mg/L.
Groundwater in the area is in equilibrium respect to calcite and amorphous silica and oversaturated with respect to most of the silicate minerals forming the aquifer. The solution is subsaturated in sulfate and halogen salts, which explains the absence of gypsum or mirabilite in the sediment. The cation adsorption selectivity determined for normal flow conditions from recharge to discharge zones is Ca>Mg>Na>K and is responsible of the evolution from calcium waters to sodium waters, and the high CEC ofthe loess-like sediments in the Argentine Pampa enhance the importance of the process. Incongruent dissolution of silicates with montmorrillonite neoformation is also partially responsible of silica and calcium content.
Acknowledgements
The authors are grateful to Cart. Virginia Bernasconi and Cart. Marcelo Farenga for the figures illustrating the text, to Tec. Mariquita Trassens for the textural analysis of sediments and to Tec. Angel Ferrante for his collaboration in sampling campaigns.
References
1. F. Ameghino. "La formación Pampeana o estudio de los terrenos de transporte de la cuenca del Plata". Buenos Aires-París. 1881. pp. 371. [ Links ]
2. C. Schultz, E. Castro. "Estudio, planificación y explotación del agua subterránea. Una trilogía utópica en la República Argentina". III Congreso Argentino de Hidrogeología. Actas I: 219-225. Rosario, Argentina. 2003. [ Links ]
3. H. Nicolli, J. Suriano, M. Gómez, L. Ferpozzi, O. Baleani. "Groundwater contamination with arsenic and other trace elements in an area of the Pampa, province of Córdoba, Argentina". Environmental Geology. Vol. 14. 1989. pp. 3-16. [ Links ]
4. H. Nicolli, A. Tineo, J. Garcia, C. Falcón. Distribución del arsénico y otros oligoelementos asociados en aguas subterráneas de la región de Los Pereyra. Provincia de Tucumán, Argentina. En: Galindo G, Fernández- Turiel JL, Parada MA, Gimeno Torrente D, eds. Arsénico en aguas: origen, movilidad y tratamiento. Taller. II Seminario Hispano-Latinoamericano sobre temas actuales de hidrología subterránea - IV Congreso Hidrogeológico Argentino. 2005 Octubre 25-28; Rio Cuarto, Argentina. 2005. pp. 83-91. [ Links ]
5. H. Nicolli, J. Bundschuh, M. Blanco, O. Tujchneider, H. Panarello, C. Dapeña, J. Rusansky. "Arsenic and associated trace-elements in groundwater from the Chaco-Pampean plain, Argentina: Results from 100 years of research". Science of the Total Environment. Vol. 429. 2012. pp. 36-56. [ Links ]
6. E. Kruse, J. Ainchil. "Assessment of fluoride concentration in groundwater, Saldungaray, Argentina". Sililo et al (editors): Groundwater: Past Achievements and Future Challenges. Proceedings of the XXX IAH Congress. Rotterdam, Netherlands. 2000. pp. 545-548. [ Links ]
7. J. Bundschuh, B. Farias , R. Martin , A. Storniolo , P. Bhattacharya , J. Cortes , G. Bonorino , R. Alboury. "Groundwater arsenic in the Chaco-Pampean Plain, Argentina: Case study from Robles County, Santiago del Estero Province". Appl. Geochem. Vol.19. 2004. pp. 231-243 [ Links ]
8. M. Gomez, M. Blarasin, D. Martinez. "Arsenic and Fluoride in a loess aquifer in the central area of Argentina". Environmental Geology. Vol. 57. 2009. pp. 143-155. [ Links ]
9. D. Martinez, O. Quiroz, H. Massone, P. Palacio, L. Lima. "Hydrogeochemistry of Fluoride in the Quequen River Basin: Natural Pollutants Distribution in the Argentine Pampa". Environmental Earth Sciences. Vol. 65. 2012. pp. 411-420. [ Links ]
10. J. Costa, H. Massone, D. Martinez, E. Suero, F. Bedmar. "Nitrate contamination of a rural aquifer and nitrate accumulation in the no saturated zone". Agricultural-Water Management. Vol. 57. 2002. pp. 33-47. [ Links ]
11. I. Chebotarev. "Metamorphism of Natural Waters in the Crust of Weathering". Geochim Cosmochim. Acta. Vol. 8. 1955. pp. 22-48,137. [ Links ]
12. R. Garrels, C. Christ. Solutions, Minerals and Equilibrio. Ed. Harper & Row. New York, USA. 1965. pp. 450. [ Links ]
13. E. Bocanegra, H. Massone, D. Martinez, E. Civit, M. Farenga. "Groundwater contamination: risk management and assessment for landfills in Mar del Plata, Argentina". Environmental Geology. Vol. 40. 2001. pp. 732-741. [ Links ]
14. D. Martinez, H. Massone, J. Ceron, M. Farenga, A. Ferrante. "Hidrogeoquímica del área de disposición final de residuos de Mar del Plata, Argentina". Boletín Geológico Minero de España, Número Monográfico Iberoamericano Hidrología Subterránea. Vol. 114. 2003. pp. 237-246. [ Links ]
15. D. Martinez, S. Mascioli, E. Bocanegra. "Determination of Zn partition coefficient and reactive transport simulation form landfills in Mar del Plata, Argentina". Environmental Geology. Vol. 51. 2006. pp. 463-469. [ Links ]
16. M. Teruggi. "The nature and origin of the Argentine loess". Journal of Sedimentary Petrology. Vol. 27. 1957. pp. 322-332. [ Links ]
17. J. Tricart. "Geomorfología de la Pampa Deprimida". Instituto Nacional de Tecnología Agropecuaria, Colección Científica N° 12. Buenos Aires, Argentina. 1973. pp. 202. [ Links ]
18. M. Currell, I. Cartwright. "Major-ion chemistry, S13C and S7Sr/S6Sr as indicators of hydrochemical evolution and sources of salinity in groundwater in the Yuncheng Basin, China". Hydrogeology Journal. Vol. 19. 2011. pp. S35-S50. [ Links ]
19. C. Thornthwaite. "An approach toward a rational classification of climate". Geogr. Rev. Vol. 3S. 194S. pp. 55-94. [ Links ]
20. Dalla, M. Iñiguez. "La Tinta, Precambrico y Paleozoico de Buenos Aires". VII Cong. Geol. Arg. Actas, I. La Plata, Argentina. 1979. pp. 539-550. [ Links ]
21. M. Teruggi, J. Kilmurray. "Tandilia relatorio geología de la Provincia de Buenos Aires". VI Congr Geol Argentino. Bahía Blanca, Argentina. 1975. pp. 55-77. [ Links ]
22. J. Sala. "Recursos Hídricos (especial mención de las aguas subterráneas)". Relatorio Geología de la Provincia de Buenos Aires. IV Congreso Geológico Argentino. Buenos Aires, Argentina. 1975. pp. 169. [ Links ]
23. C. Appelo, D. Postma. Geochemistry, Groundwater and Pollution. Ed. A.A. Balkema/Rotterdam/ Brookfield. Leiden, The Netherlands. 1993. pp. 536. [ Links ]
24. L. Plummer, E. Prestemon, D. Parkhurst. "An Interactive Code (NETPATH) for Modelling NET Geochemical Reactions Along a Flow PATH." U.S.G.S. Water Resources Investigations Report 912- 407S. Reston, VA, USA. 407S, 1991. pp. 227. [ Links ]
25. D. Parkhurst, T. Appelo. "User's guide to PHREEQC (Version 2) - A computer program for speciation, batch reaction, one dimensional transport, and inverse geochemical calculations. U.S.G.S." Water-Resources Investigations Report 99-4259. Reston, VA, Estados Unidos. 1999. pp. 1-312. [ Links ]
26. A. Walkey, A. Black. Organic carbon. Black CA (editor). Methods of soil analysis. American Society of Agronomy. Madison, Wisconsin, USA. 1965. pp. 1372-1374. [ Links ]
27. T. Kent, E. Payne. Sampling Groundwater Monitoring Wells: Special Quality Assurance and Quality Considerations. L.H. Keith (editors) .Principles of Environmental Sampling. ACS Professional Reference Book. Washington DC, USA. 1988. pp. 231-246. [ Links ]
28. E. Brown, M. Skougstad, M. Fishman. "Methods for collection and analysis of water samples for dissolved minerals and gases". US Geol Surv Tech Water Resour Invest 5 (A-1). Washington DC, USA. 1970. pp. 160. [ Links ]
29. R. Folk, W. Ward. "Brazos River bar: a study in the significance of grain size parameters". Journal of Sedimentary Petrology. Vol. 27. 1957. pp. 3-26. [ Links ]
30. T. Henderson. "Geochemistry of groundwater in two sandstone aquifer systems in the Northern Great Plains in parts of Montana, Wyoming, North Dakota and South Dakota". U.S.G.S. Professional Paper, 1402C. Washington, USA. 1984. pp. 93. [ Links ]
31. M. Tardy. "Characterization of principal weathering types by the geochemestry of waters from some European and African cristalline massifs". Chem. Geology. Vol. 7. 1971. pp. 253-271. [ Links ]
32. R. Berner. "Chemical diagenesis in some modern carbonate sediments". American Journal of Science. Vol. 264. 1966. pp.2-36. [ Links ]
33. W. Marshall, J. Warakomski. "Amorphous silica solubilities - II. Effect of aqueous salt solutions at 25°C. Geochimica Cosmochimica Acta. Vol. 44. 1980. pp. 915-924. [ Links ]
34. D. Martinez, E. Bocanegra. "Hydrogeochemistry and cationic exchange processes in the coastal aquifer of Mar del Plata, Argentina". Hydrogeology Journal. Vol. 10. 2002. pp. 393-408. [ Links ]