Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230
Rev.fac.ing.univ. Antioquia no.67 Medellín Apr./June 2013
ARTÍCULO ORIGINAL
Synthesis of two new Nickel and Copper- Nickel vanadates used for propane oxidative dehydrogenation
Síntesis de dos nuevos vanadatos de Níquel y Cobre-Níquel usados en la deshidrogenación oxidativa de propano
Juan Pablo Hernández1, Adriana Echavarría1*, Luz Amparo Palacio1,2
1Grupo Catalizadores y Adsorbentes. Universidad de Antioquia. Calle 67 # 53 - 108. Bloque 1 - 314. Medellín, Colombia.
2Instituto de Química. Universidade do Estado do Rio de Janeiro. Rua Sao Franciso Xavier, 524. Rio de Janeiro, Brazil.
*Autor de correspondencia: teléfono: + 54 + 1 + 2195666, correo electrónico: aechavar@udea.edu.co (A. Echavarría)
(Recibido el 20 de Febrero de 2012. Aceptado el 26 de abril de 2013)
Abstract
Two new vanadates have been successfully synthesized by the hydrothermal and coprecipitation methods. Both vanadates were calcined at 600 °C and the resulting catalysts were tested on reaction of oxidative dehydrogenation of propane. The catalysts were characterized by x-ray diffraction, atomic absorption, thermogravimetric analysis, and differential temperature analysis. The reaction was carried out in the temperature range of 350-500 °C. A conversion of propane of 10.6 % and a selectivity towards propene of 29.9 % at 400 °C were obtained with nickel vanadate; a conversion of 1.9 % and a selectivity of 56.9 % were reached at the same temperature with the nickel copper vanadate.
Keywords:Oxidative dehydrogenation, nickel vanadate, copper-nickel vanadate, propane, propene
Resumen
Se probaron dos nuevos vanadatos en la deshidrogenación oxidativa de propano. Los catalizadores fueron sintetizados por el método hidrotérmico y de coprecipitación, la caracterización se llevó a cabo por medio de difracción de rayos X, absorción atómica, análisis termogravimétrico y análisis térmico diferencial. La reacción se realizó en un rango de temperatura de 350-500 °C. Con el vanadato de níquel se obtuvo una conversión de propano de 10.6 % y una selectividad hacia propeno del 29.9 % a 400 °C y con el vanadato de cobre y níquel se alcanzó a la misma temperatura una conversión de 1.9 % y una selectividad del 56.9 %.
Palabras clave: Deshidrogenación oxidativa, vanadato de niquel, vanadato de cobre-niquel, propano, propeno
Introduction
In recent years, the study of Oxidative Dehydrogenation (ODH) of alkanes has gained importance due to the high demand for light alkenes in plastic industries, and because of the way it may be used with natural gas in order to enhance its content in light alkanes. The ODH is an alternative route for the production of alkenes such as propene, since coke production does not occur, and there is no equilibrium limitation [1].
The highest yields achieved in the oxidative dehydrogenation of propane have been given with zinc and magnesium vanadates [2], K/Mo catalysts supported on mixed oxides of silicon and titanium [3], oxides of alkaline earth metals [4, 5] and an Ni(Co)MoO4 trimetallic catalyst [6], among others. However, these catalysts yields do not exceed 24 %. Different factors make a catalyst useful in the oxidative dehydrogenation reaction, such as the nature of active oxygen species, the redox, and the acidic properties [1].
The magnesium vanadates are among the catalysts which have improved performance in these reactions [1-7]. The catalytic experiments made with these catalysts suggest that the high activity may be related to the easy removal of vanadates surface oxygen groups [8-11]; consequently, vanadium is found in many formulations of catalysts for oxidative dehydrogenation.
Different vanadium-based catalysts have been tested, either in bulk or supported form, mixed with calcium, nickel ,and copper, such as: V-Ca-O [12] with a conversion of 5 % and a selectivity of 48 %; hydroxyapatite calcium replaced with vanadium [13], reaching a conversion of 17.2 % and a selectivity of 52.4 % at 450 °C; calcium magnesium vanadates [9], with conversion of 12.5 % and selectivity of 39.3 % at 450 °C; copper magnesium vanadates [10], with a conversion of 9.3 % and a selectivity of 32.9 % at 450 °C; and finally, nickel vanadates [14] with a conversion of 19.45 % and a selectivity of 49.9 % at 425 °C.
In this work, two new nickel and copper-nickel vanadates were synthesized by the hydrothermal and coprecipitation methods, this latter allows saving energy in the process of synthesis compared with other methods used in previous work [918] for similar materials. The characterization of and their catalytic activity in the oxidative dehydrogenation of propane has been reported.
Experimental
Preparation of catalysts
The precursors were first synthesized by the hydrothermal and coprecipitation methods [15], then calcined at 600 °C for 3 hours at 5 °C/min to obtain the final catalyst. In the hydrothermal synthesis, the solutions are mixed and the mixture is heated in lined-autoclave at a fixed temperature and autogen pressure. In the coprecipitation method, the mixture is kept at room temperature for a fixed time. Each precursor was prepared from individual solutions of vanadium, NH4VO3 (Merck 99 %), to which copper, CuCl2-2H2O (Merck 99 %), and/or nickel, NiCl2-6H2O (Merck 98 %), solutions were added. The precipitating agent used was NH4OH (Merck 28-30 %) and the heating temperature for the hydrothermal method was 100 °C, as shown in table 1. The crystallization time varied between 72 and 96 hours. The solids precursors were called NiV and CuNiV. Calcined precursors (final catalysts) were labelled by adding the calcination temperature to each one of them (NiV600 and CuNiV600).
Characterization of the catalysts
The chemical composition of V, Cu and Ni of the two new materials was determined by atomic absorption on a Unicam Solaar. The identification of crystalline phases was carried out through the diffractograms obtained in a Rigaku Miniflex apparatus with radiation Cukα (λ = 1.5418 Å) operated at 40 kV and 30 mA and with the aid of the PDF-2 diffraction database (Powder Diffraction File). The thermogravimetric analysis (TGA) was performed in a TA Instruments Hi-Res TGA 2920 and the differential thermal analysis (DTA) was performed on a TA Instruments DSC 2920, both in a temperature range from 30 to 800 °C, 10 °C/min, under air atmosphere.
Indexing of the unit cell
The x-ray data for indexing were obtained in a Bruker diffractometer, equipped with a graphite monochromator, using radiation Cukα (λ = 1,5406 Å) operated at 40 kV and 40 mA, with a scan of 10 to 60° in 2θ, a step of 0.02°/step and a time per step of 5 seconds. The pattern decomposition was carried out using WINPLOT; the peaks found were analyzed using the CRYSFIRE, ITO, and TREOR algorithms. The refinement of the cell and the determination of the space group were performed with CHEKCELL.
Catalytic tests
The oxidative dehydrogenation of propane was carried out in a quartz reactor (i.d 5 millimeters, length 400 millimeters) at atmospheric pressure in a temperature range between 350-500 °C and a space velocity of 100 ml g-1 min-1, using 0.2 grams of catalyst. The feed that contained 29 % propane and 71 % air, corresponding to a molar ratio of propane/ air = 2. The reaction products were analyzed online by gas chromatography on Shimadzu GC-9A with thermal conductivity detector, using a 5A molecular sieve and Porapak Q columns.
Results and discussion
Characterization of catalysts
Precursors and materials obtained from calcinations were identified by x-ray diffraction. The diffractograms as a function of calcination temperature are shown in figure 1, and the identification of the crystalline phases present in the catalyst (the precursor calcined at 600 °C) and the precursor are shown in table 2.
The nickel and nickel-copper vanadates could not be found in the database consulted, which suggests a development of two possible new materials; however the XRD pattern is similar to fz, which has been obtained for other crystalline vanadate phases, but combined with zinc or copper [16-19]. The nickel catalyst (calcined precursor) was identified as Ni3V2O8, and the nickel and copper ones as a mixture of Cu3V2O8 and Ni3V2O8.
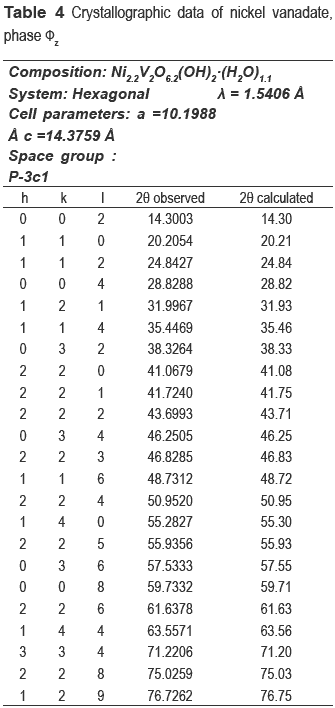
The compositions of metals in the new precursors were analyzed and the amount of nickel was 33.1 % for NiV and 14.81 % for the CuNiV, the copper amount for the latter was 14.94 %. As demonstrated by XRD, the two new materials have the same structure of phase fz, whose general formula for zinc vanadate is Zn3(OH)2V2O7.2H2O [15]. In our case, this formula should be maintained, but the divalent metal will not be Zn but Ni and/or Cu. Taking into account the proposed theoretical formula M3-x(OH)2V2O7-x.nH2O, where M: Ni or a mixture of Cu and Ni and n the number of hydration water molecules) for a fy phase and the chemical analysis, the following formulas Ni2.2(OH)2V2O6.2.1.1H2O for NiV, and Ni0.63Cu0.59(OH)2V2O5.22.0.9H2O for CuNiV were established.
These formulas agree quite well with experimentally obtained results, because the theoretical percentages of Ni and H2O in NiV is 33.6 % and 9.8 %, respectively (9.8 %, experimental of H2O); for CuNiV the theoretical percentages of Ni and Cu are respectively 15.7 % and 15.8 % and H2O is 9.9 % (9.9 %, experimental of H2O).
Thermograms of the precursors (figure 2) show a weight loss up to 100 °C, which is associated with humidity in the material.
Figure 2a shows a weight loss of 5.2 % accompanied by an endothermic event between 100-250 °C, which is due to desorption of hydration water. There is an endothermic event with a weight loss of 4.61 %, associated to the OH- present in the structure between 250 and 500 °C; the percentage of theoretical mass loss is equal to that found experimentally, with a value of 9.8 %, which provides the following thermal decomposition reaction:
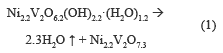
Figure 2b shows two endothermic events, first at 200-250 °C, where weight loss is 8 %, associated with the desorption of hydration water, and the second in a range at 250-400 °C, accompanied by a weight loss of 4 %, assigned to the release of water from the structure hydroxyl. The theoretical total weight loss was 9.9 %, which was equal to experimentally obtained. The following thermal decomposition reactions were proposed:
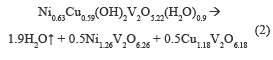
The decomposition reactions for the NiV and CuNiV precursors show that the mixed oxides formed after calcination are not exactly the same to those found with the identification by XRD, since they are not stoichiometric.
Based on the thermal behavior of precursors, it was decided to calcine them at 600 °C, temperature at which there is no further loss of mass or phase change.
Indexing
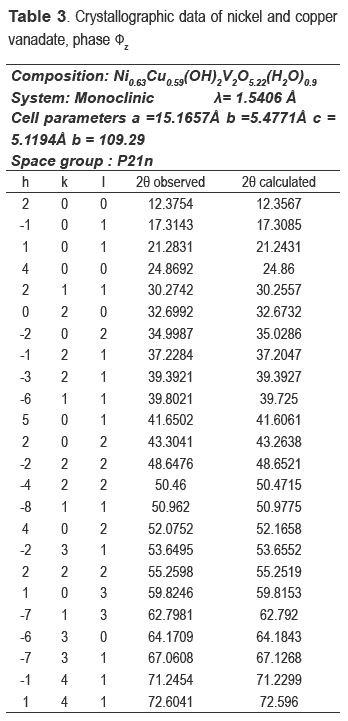
The precise determination of the positions of the peaks in the X-ray pattern was carried out using the WINPLOT program. Obtained reflexions were used as input for the CRYSFIRE program. To find the best cell for the NiCuV, the reflections were tested in the CHEKCELL program. The hexagonal cell was initially tested, but two lines were not indexed. Orthorhombic cells were indexed, but their figure of merit was very low.
With cells in the triclinic system, all reflections were not indexed. The best solution was the monoclinic cell, with a figure of merit 14 and I20 of 20. In the case of NiV material, a similar procedure of CuNiV was followed. The data given for CRYSFIRE were analyzed and checked with the CHEKCELL program. One of the selected results showed a figure of merit of 20, but they found cell parameters were divided by 2, because the unit cell showed a very large volume (V = 1298 Å3) if these values are compared with the phase  z. However, this first analysis did not work, since not all lines were indexed, then, the cells reported by Zhang [19] and Hoyos [17], who worked with similar materials, were tested. The first assay was not good. Finally, the cell was refined and inscribed in the hexagonal system.
z. However, this first analysis did not work, since not all lines were indexed, then, the cells reported by Zhang [19] and Hoyos [17], who worked with similar materials, were tested. The first assay was not good. Finally, the cell was refined and inscribed in the hexagonal system.
The refinement of the cell and the space group determination were carried out with CHECKCELL. Tables 3 and 4 show the crystallographic data of new materials.
Catalytic Activity
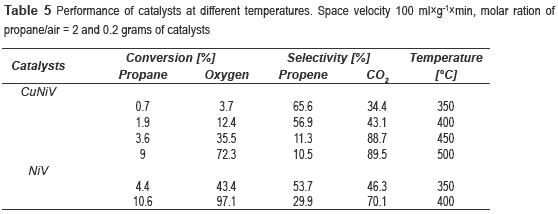
The catalytic behaviour of the vanadates in the reaction of oxidative dehydrogenation of propane was studied; the products were propylene and CO2. Table 5 shows the variation of conversion and selectivity with temperature for the catalysts. It can be seen that as the temperature increases so does the conversion, but the selectivity decreases dramatically, it could be a consequence the temperature favors the kinetics of CO2 yields. With the NiV catalyst was not possible to test above 400 °C due to the inability to control the inflow, possibly for excessive coke deposition and subsequent plugging of output of the reactor.
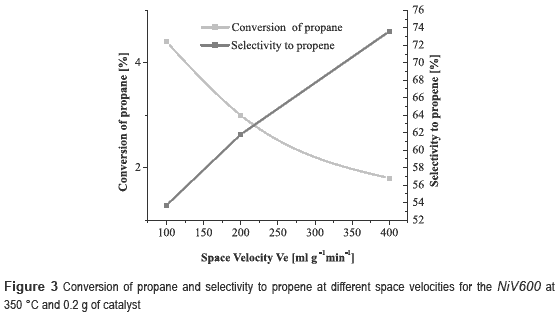
It can be noticed that the best performance is achieved with the NiV, with a conversion of propane of 10.6 % and selectivity to propene of 29.9 %. However, the catalyst most selective to propene was the nickel and copper trimetallic catalyst. Since NiV600 catalyst showed the best results, other experiments were conducted at different space velocities, 100, 200 and 400 ml g-1 min-1, in order to observe its effect on the reaction. It was decided to work at 350 °C, because at this temperature was obtained the highest selectivity.
From figure 3 it can observed that when the space velocity increases, the conversion of propane decreases and the selectivity to propene increases, as expected, because at high space velocity there is less contact time and therefore there is less possibility to occur side reactions how the CO2 production. There is also a decrease in catalytic activity due to the short time that the propane molecule is in contact with the catalyst.
Conclusions
Two new materials  z type, comprised from vanadium-nickel and vanadium-nickel-copper, with the formula Ni2.2V2O6.2(OH)2.(H2O)1.1 and (Ni0.52Cu0.48)1.22(OH)2V2O5.22.H2O0.9, respectively, were synthesized by the hydrothermal and coprecipitation methods. These materials were calcined at 600 °C in order to obtain catalysts for the propane oxidative dehydrogenation reaction. The best performance was obtained with the NiV600 (conversion of propane of 10.6 % and selectivity to propene of 29.9 % at 400 °C). The low conversion obtained with Cu catalyst could be attributed to low accessibility to the vanadium sites (the active site), occurred by masking with copper atoms.
z type, comprised from vanadium-nickel and vanadium-nickel-copper, with the formula Ni2.2V2O6.2(OH)2.(H2O)1.1 and (Ni0.52Cu0.48)1.22(OH)2V2O5.22.H2O0.9, respectively, were synthesized by the hydrothermal and coprecipitation methods. These materials were calcined at 600 °C in order to obtain catalysts for the propane oxidative dehydrogenation reaction. The best performance was obtained with the NiV600 (conversion of propane of 10.6 % and selectivity to propene of 29.9 % at 400 °C). The low conversion obtained with Cu catalyst could be attributed to low accessibility to the vanadium sites (the active site), occurred by masking with copper atoms.
The precursor materials identified as NiV and CuNiV showed a change in their composition and structure to be calcined; leading to formation of metastable phases, which are more dense than initial precursor, as are shown with the X-ray diffraction identification.
The catalyst (NiV600) showed good activity, but it is remarkable that the method of preparation of the precursor is much better than reported by Zhaorigetua et. al [14] for other Zn-V catalyst, in terms of saving energy.
References
1. F. Cavani, N. Ballarini, A. Cericola. ''Oxidative dehydrogenation of ethane and propane: How far from commercial implementation?''. Catal. Today. Vol. 127. 2007. pp. 113-131. [ Links ]
2. H. Kung, M. Chaar. ''Oxidative Dehydrogenation of Alkanes to Unsaturated Hydrocarbons''. U.S. Patent N.° 4,777,319. January 01. 1988. [ Links ]
3. U. Ozkan, R. Watson. ''Preparation and use of a catalyst for the oxidative dehydrogenation of lower alkanes''. U.S. Patent N. ° 6,521,808. Februrary 18. 2003. [ Links ]
4. L. Levels, S. Fuchs, K. Seshan, J. Lercher, L. Lefferts. ''Oxidative conversion of light alkanes to olefins over alkali promoted oxide catalysts''. Appl. Catal. A. Vol. 227. 2002. pp. 287-297. [ Links ]
5. L. Leveles, K. Seshan, J. lercher, L. Lefferts. ''Oxidative conversion of propane over lithium-promoted magnesia catalyst - I. Kinetics and mechanism''. J. Catal. Vol. 218. 2003. pp. 296-306. [ Links ]
6. L. Madeira, M. Portela, C. Mazzocchia. ''Nickel molybdate catalysts and their use in the selective oxidation of hydrocarbons''. Catal. Rev. Vol. 46. 2004. pp. 53-110. [ Links ]
7. E. Heracleous, M. Machli, A. Angeliki. ''Oxidative dehydrogenation of ethane and propane over vanadia and molybdena supported catalysts''. J. Mol. Catal. A. Vol. 232. 2005. pp. 29-39. [ Links ]
8. S. Sugiyama, T. Hashimoto, N. Shigemoto, H. Hayashi, ''Redox Behaviors of Magnesium Vanadate Catalysts During the Oxidative Dehydrogenation of Propane''. Catal. Lett. Vol. 89. 2003. pp. 229-233. [ Links ]
9. S. Sugiyama, T. Hashimoto, Y. Morishita, N. Shigemoto, H. Hayashi. ''Effects of calcium cations incorporated into magnesium vanadates on the redox behaviors and the catalytic activities for the oxidative dehydrogenation of propane''. Appl. Catal. A. Vol. 270. 2004. pp. 253-260. [ Links ]
10. S. Sugiyama, T. Hashimoto, Y. Tanabe, N. Shigemoto, H. Hayashi. ''Effects of the enhancement of the abstraction of lattice oxygen from magnesium vanadates incorporated with copper(II) cations on the oxidative dehydrogenation of propane''. J. Mol. Catal. A: Chem. Vol. 227. 2005. pp. 255-261. [ Links ]
11. S. Sugiyama, T. Osaka, Y. Hirata, K. Sotowa. ''Enhancement of the activity for oxidative dehydrogenation of propane on calcium hydroxyapatite substituted with vanadato''. Appl. Catal. A. Vol. 312. 2006. pp. 52-58. [ Links ]
12. R. Valenzuela, V. Cortes. ''On the intrinsic activity of vanadium centres in the oxidative dehydrogenation of propane over V-Ca-O and V-Mg-O catalysts''. Topics in Catal. Vol. 11-12. 2000. pp. 153-160. [ Links ]
13. S. Sugiyama, T. Osaka, T. Hashimoto, K. Sotowa. ''Oxidative Dehydrogenation of Propane on Calcium Hydroxyapatites Partially Substituted with Vanadate''. Catal. Lett. Vol. 103. 2005. pp. 121-123. [ Links ]
14. B. Zhaorigetua, W. Lib, H. Xub, R. Kiefferc. ''Correlation Between the Characteristics and Catalytic Performance of Ni-V-O Catalysts in Oxidative Dehydrogenation of Propane''. Catal. Lett. Vol. 94. 2004. pp. 125-129. [ Links ]
15. L. Palacio. ''Métodos de síntesis de nuevos materiales basados en metales de transición''. Rev. Fac. Ing. No. 22. 2004. pp. 51-61. [ Links ]
16. M. Khaled, B. Bouzid, A. Yahya. ''Room temperature synthesis of zinc pyrovanadate Zn3(OH)2V2O7.2H2O''. J. Mater. Chem. Vol. 9. 1999. pp. 1543-1545. [ Links ]
17. D. Hoyos, A. Echavarria, C. Saldarriaga. ''Synthesis and structure of a porous zinc vanadate, Zn3(VO4)2.3H2O''. J. Mater. Sci. Vol. 36. 2001. pp. 5515-5518. [ Links ]
18. L. Palacio, J. Silva, F. Ribeiro, M. Ribeiro. ''Catalytic oxidation of volatile organic compounds with a new precursor type copper vanadato''. Catal. Today. Vol. 133. 2008. pp. 502-508. [ Links ]
19. F. Zhang, P. Zavalij, M. Whittingham. ''Synthesis and characterization of a pipe-structure manganese vanadium oxide by hydrothermal reaction''. J. Mater. Chem. Vol. 9. 1999. pp. 3137- 3140. [ Links ]