Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230
Rev.fac.ing.univ. Antioquia no.68 Medellín July/Sept. 2013
ARTÍCULO ORIGINAL
Effect of chloride salts on biodesulfurization process of a colombian coal
Efecto de sales cloruros en un proceso de biodesulfurización de un carbón colombiano
Gerardo Andrés Caicedo Pineda1, Marco Antonio Márquez Godoy2
1M.Sc. Biotechnology. National University of Colombia. Cra. 80 No. 65-223. Medellin, Colombia.
2Ph.D. in Applied Mineralogy, School of Materials Engineering. National University of Colombia. Cra. 80 No. 65-223. Medellin, Colombia.
*Autor de correspondencia: teléfono: + 57 + 315 802 37 60, fax: + 57 + 4 + 425 52 48, correo electrónico: gacaiced@unal,edu.co (G. Caicedo)
(Recibido el 26 de marzo de 2012. Aprobado el 5 de agosto de 2013)
Abstract
A chloride salts medium was evaluated in biodesulfurization processes of a Colombian coal (2.34% total sulfur: 1.34% as pyritic, 0.90% as organic and 0.10% from sulfates), employing a consortium of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. The process was carried out at three pulp concentrations (9.09%, 16.67% and 23.08%) and was compared with assays under similar conditions but containing modified T&K medium. All the tests were determined by measuring changes in soluble iron, pH, redox potential and microbial populations approach. Original and treated coal was analyzed by scanning electron microscopy with an energy-dispersive X-ray (SEM-EDX). For bioassays with chloride salts medium, the results showed above 70% of pyritic sulfur oxidation after 12 days, regardless of pulp concentration in contrast with results obtained for bioassays with a T&K modified medium where pulp concentration had influence and the pyrite oxidation was lower (below 55%).
Keywords: Acidithiobacillus, biodesulfurization, coal, iron removed, pyrite, culture medium
Resumen
Se evaluó el efecto de un medio de sales cloruros para un consorcio de Acidithiobacillus ferrooxidans y Acidithiobacillus thiooxidans en un proceso de biodesulfurización de un carbón colombiano (azufre total: 2.34%, azufre pirítico: 1.34%, azufre orgánico: 0.90% y azufre de sulfatos: 0.10%), bajo tres concentraciones de pulpa (9.09%, 16.67% y 23.08%). Los ensayos se compararon con otros bajo condiciones similares, pero utilizando medio T&K modificado. Todos los procesos se monitorearon con mediciones periódicas de hierro en solución, pH, potencial redox y concentración celular. El carbón fue analizado antes y después de la biodesulfurización por microscopía electrónica de barrido con analizador microquímico (SEM-EDX). Los ensayos con el medio de sales cloruros obtuvieron una oxidación del 70% de azufre pirítico después de 12 días para todas las concentraciones de pulpa evaluadas. En contraste, en los ensayos con el medio T&K modificado, la concentración de pulpa influyó en la oxidación de pirita y fue menor (por debajo de 55%).
Palabras clave: Acidithiobacillus, biodesulfurización, carbón, hierro removido, pirita, medio de cultivo
Introduction
Precombustion processes to reduce sulfur forms from coal (organic and inorganic) are considered good methods to limit sulfur oxide emissions [1, 2]. Among the different kinds of desulfurization processes, the biological have many advantages in comparison to chemical and physical processes. Coal biodesulfurization has lower operational costs and is easily designed and built, does not require high temperatures or pressures for its operation, self- regenerate solvents in form of ferric iron (implied in pyrite oxidation) and produce no pollutant gases. Also, liquid and solid wastes are easily treated and environmentally accepted [3-8]. Although some researchers have designed plants at industrial level, the commercialization of this process has not been enhanced yet, especially related to operation cost versus residence time [7]. Many physic-chemical factors must be enhanced yet.
Biodesulfurization processes are based on bioleaching mechanisms, where sulfide oxidation is catalyzed by acidophilic microorganisms in an aqueous medium, generating soluble sulfates [911]. Physical, chemical and biological factors as pH, dissolved oxygen (DO), temperature, iron concentration, number and type the microorganisms have been evaluated, searching alternatives for a possible application at commercial level [12-15].
Sometimes, sulfates produced during pyrite oxidation precipitate onto the surface of coal, forming insoluble salts, jarosites and others, which can reduce desulfurization efficiency [15]. An alternative to avoid sulfate precipitation involves decreasing sulfate concentration in the culture media. It implies to reduce the concentration of sulfate salts used as nutrients or change them by their counterparts in another kind of salts (chloride, nitrate, phosphate). However, it is important to take into account that new reagents do not affect cell growth and helps to increase the efficiency of sulfur removal.
This work evaluated the effect of two culture media (a conventional T&K medium and an experimental using chloride salts) on pyrite biooxidation and sulfate precipitation, in order to obtain a basis for selecting conditions which provide a biological alternative for the use of sulfur-rich coals.
Experimental
Coal
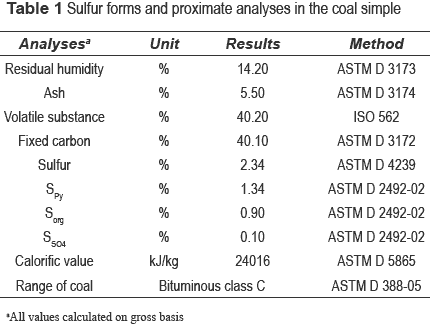
Coal sample was collected from ''La Guacamaya'' coal mine (Puerto Libertador, Córdoba, Colombia). Proximate analyses and sulfur forms are shown in table 1. The sample was grounded to achieve a particle size -60 Tyler mesh. The mineralogical composition of the sample was established by Scanning Electron Microscopy with Energy Dispersive X-Ray Spectrometer (SEM/EDS).
Microorganisms
A consortium which contains Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans was selected from The Laboratory of Biomineralogy of The National University of Colombia. The culture was previously adapted to coal biode sulfurization according to an established protocol [16]. An inoculum was prepared in 500 mL flasks, with a working volume of 200 mL, using two different media: modified T&K defined as Tk (g/L): (NH4)2SO4, 0.5 g/L; MgSO4, 0.5 g/L; KH2PO4, 0.5 g/L; S, 0.1% w/v; FeSO4.7H2O, 1.0 and chloride salts medium defined as Cl (g/L): NH4Cl, 0.5; MgCl2, 0.5; KH2PO4, 0.5; S, 0.1% w/v; FeSO4.7H2O, 1.0. To each medium was added 10 %w coal and 10 %v of inoculum with a bacterial concentration between 107-108 cells/ mL. Initial pH was adjusted to 1.5. The cultures were incubated in a shaker for 12 days at 30 °C ± 1 °C, using a mixing rate of 180 ± 2 rpm.
Coal biodesulfurization process
Formal assays were prepared in 500 mL flasks (working volume 200 mL). The variables to evaluate were: (i) media: Tk and Cl and (ii) pulp concentrations (g of coal:mL leaching solution): 1:10 (9.09 %w), 2:10 (16,67 %w) and 3:10 (23,08 %w). Initial pH was adjusted to 1.5 with sulfuric acid. All processes were incubated for 12 days under similar conditions of the inoculum preparation. All the experiments had a respective replica and abiotic control (i.e., an essay with the same operation conditions but without inoculum).
The assays were monitored every two days measuring pH and redox potential (ORP), using a pH/ORP-meter SCHOTT Handylab. Microorganism concentration was determined by cell count in Neubaüer chamber. Total iron in solution was determined in a spectrophotometer Thermo GENESYS UV 10, employing the method 3500-Fe B, according to the Standard Methods for Water Analyses.
Iron removed from coal (Felix, g Fe/kg coal) was calculated by equation (1) in base to iron in solution at time 0 (Fe0, mg/L), iron in solution at time t (Fet, mg/L), leachate volume (V, L) and coal employed (Mcoal, g).
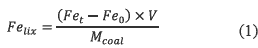
At the end of the experiments, sulfur forms in coal samples were measured by the ASTM D 2492-02 method. The mineralogical composition of the treated samples was established by SEM/ EDS.
Results and discussion
Mineralogical characterization of coal
Observations done over pyrite crystals in the SEM/EDS showed that pyrite is mainly present as framboidals surrounded by disseminate crystals (figure 1.a) and aggregates (figure 1.b). Pyrite near to grains boundary (figure 1.c) and individual crystals (figure 1.d) were also observed.
Biodesulfurization process
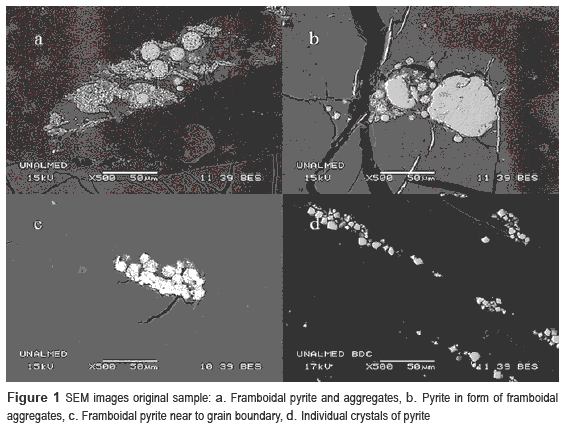
On the first days of the processes, pH values increased for all the bioassays (figure 2) and the abiotic controls. Between both culture media, the assays with Tk medium had the highest pH. After the second day, pH diminishes for all bioassays.
Abiotic controls did not have changes after pH increasing. On day 12, only the bioassays with pulp concentration of 1:10 obtained values below the initial one (around 1.45). The bioassay 3:10 Tk had the highest pH value at the end of process (2.11).
pH behavior during coal biodesulfurization was typical for this kind of processes. Although consumption of protons in the lag phase by bacteria, necessary for ferric ions generation, influence pH increasing [17, 18], the abiotic controls show that coal is the main causative of this phenomenon due to alkalinizing compounds, such as carbonates, which react with the acid of the media, similar to found in other similar works [6, 19-21]. For this reason, pH increasing is directly proportional to pulp concentration. The subsequent decrease in pH is explained by acid generation in the biooxidation of pyrite and elemental sulfur, showing that pH diminishing is only caused by bacteria effect [19, 22, 23]. At the end of the processes, acid produced was not sufficient to neutralize pH increasing on assays with high concentrations of pulp (2:10 and 3:10). However, Cl medium helps to maintain better conditions for the process, regardless of pulp concentration, between the evaluated parameters. Mildly acidic properties of chloride salts in Cl medium seem to help counter coal effects on pH increasing, in comparison to their counterpart sulfates.
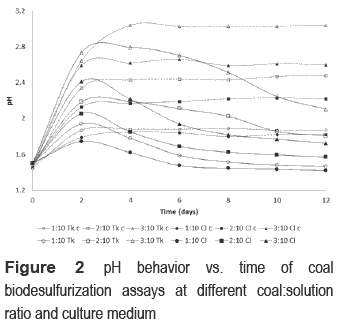
Redox potentials (figure 3) of 1:10 Cl and 1:10 Tk continuously increased until day 12. In contrast, the values of other bioassays diminished in the two first days of the process before a subsequent rising. 2:10 Cl and 3:10 Cl reached similar values that 1:10 Cl on day 4 and day 6 respectively. At the end of the process, all Cl bioassays had a similar redox potential (693 mV). The behavior of redox potential in Tk bioassays was different to the three pulp concentrations. Although 1:10 Tk reached a value of 647 mV, 2:10 Tk and 3:10 Tk had a slow increase until values below 600 mV at the end of the process. Abiotic controls did not present significant changes on redox potential in all cases.
Culture media had also influence on redox potential behavior. Whereas assays with Cl medium reached similar values around 690 mV regardless of pulp concentration, redox potential for assays with Tk medium was indirectly related to pulp concentration, according to reported by other authors in the literature [20]. Employing chloride salts, instead of sulfate salts, helps to mitigate pulp concentration effects over cell concentration.
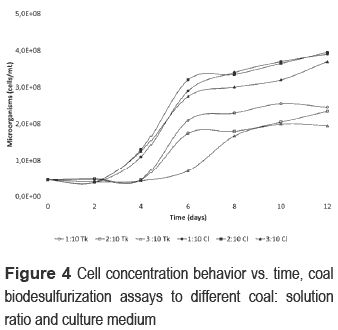
Cell concentration in leaching solution (figure 4) was different for the two media. While in the assays with Tk medium, it started growing in the fourth day, in assays with Cl medium, it started in the second day. Cl assays had a higher cell growth than Tk assays during the processes. The results obtained contrast with researches about pyrite biooxidation and cell growth under chloride salts, where the bacterial activity has a higher inhibition than using sulfate salts [24, 25]. However, in this research, Cl medium helped in the generation of a favorable environment for pyrite oxidation carried out in a biodesulfurization process. Although chloride anions may inhibit bacterial activity, also seem to minimize the inhibitory effects of coal and pulp concentration. Some authors have found that pulp concentrations above 20%w inhibit the growth of microorganism [20], similar to observed in 3:10 Tk. Nevertheless, in 3:10 Cl. cells were not affected.
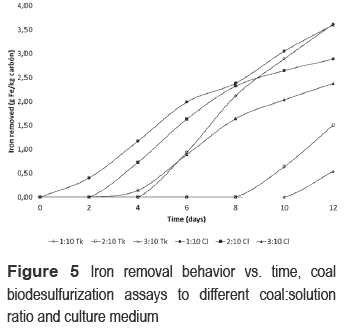
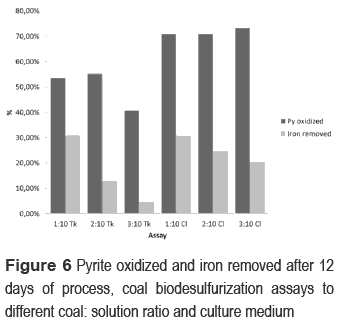
The maximum iron that can be removed from coal in all assays is 11.72 g Fe/kg coal. 1:10 Cl showed removal from the beginning of the process (figure 5), 2:10 Cl and 3:10 Cl from the second day, 1:10 Tk from fourth day, 2:10 Tk from eight day and 3:10 Tk from tenth day. At the end of the process, the better removal was reached in the bioassays with pulp concentration of 1:10 (3.6 g Fe/kg coal). Abiotic controls did not show removal of iron. After 12 days of process, bioassays with Cl medium oxidized 70% of pyrite without significant differences in this value for the three pulp concentrations (figure 6). On the other hand, in the Tk medium bioassays, the maximum reached of pyrite oxidation was around 55% except the assay 3:10 Tk (40%). Differences in iron removal were also found between the two culture media, except for pulp concentration of 1:10. Pyrite concentration did not have significant changes in abiotic controls. In base to evaluated parameters, chloride medium enhanced pyrite oxidation, which could not be affected by pulp concentration. Nonetheless, remain of organic sulfur after the biodesulfurization processes indicate the microorganism used for this work only can attack the inorganic sulfur in coal [19, 20].
SEM images after coal biodesulfurization
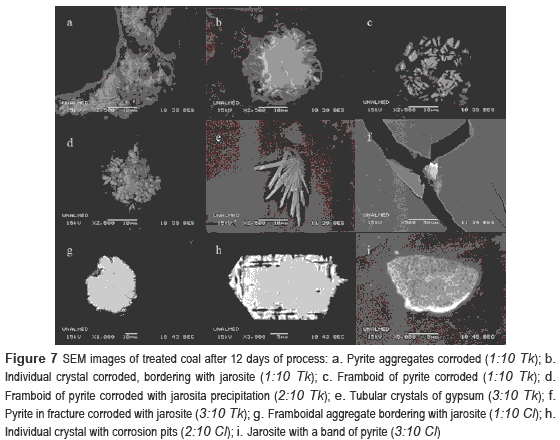
Scanning electron micrographs of biooxidated coal samples (figure 7) show typical corrosion features according to other reports on the literature [19]. In comparison to raw coal, aggregates disappear; leaving only groups of framboidals isolated which were not completely attacked. These crystals are surrounded by precipitates of hydroxisulfates. On the other hand, Individual crystals, bigger than framboidal crystals, suffered less oxidative attack during the process, showing only pits and gulfs on the surface of pyrite crystals (figure 7h). In 2:10 Tk and 3:10 Tk, tubular crystals of gypsum (figure 7e) were found.
Pyrite morphology played an important role. Size and form of the grains have a direct relation with rate of pyrite biooxidation. For this reason framboidal crystals and aggregates, which have irregular form and their pyrite grains are small (particle diameter around 0.25 μm), tends to disappear totally in comparison to individual crystals, which have well-defined-geometric form and a particle diameter even 100 larger than framboidal crystals.
The increase in the pH values generated precipitates such as gypsum and jarosites. These precipitates impede the complete oxidation ofpyrite, constituting a physical barrier for the interaction of the mineral with microorganisms and/or leaching medium [15]. Probably, the low concentration of sulfates in bioassays with Cl medium and the better conditions of pH behavior obtained may have avoided gypsum generation, helping to mitigate precipitation of products of biooxidation, increasing iron removal.
Conclusions
The bioassays performed, helped in the evaluation of factors which must be taken into account when a process of this type is carried out. Using the conventional culture medium T&K, pulp concentration influences on the rate of pyrite biooxidation and in the generation of precipitated salts (such as gypsum and jarosite). However, this factor can be mitigated employing a culture medium where sulfate salts, using as sources of nitrogen and magnesium, are replaced by their counterpart chloride salts. This salts help to maintain appropriate conditions for the growing of microorganism and action of biooxidation processes, regardless of pulp concentration.
The high content of organic sulfur in the coal sample does not permit a complete elimination of sulfur using this kind of microorganisms, because it is considered that they only can attack the inorganic sulfur in coal. Even if the process can remove the 100% of the pyritic sulfur, the organic phase would be important (0.9%). In spite of this, the fact that the predominant habit of pyrite in coal is framboidal, it is considered favorable for the biological process because the smaller size of the crystals increase the superficial area exposed to the microorganisms attack.
This work will be the support for similar researches developed in the Biomineralogy Laboratory of the National University of Colombia that pretend proposing an applicable alternative for the treatment of coals in the country.
Acknowledgements
To COLCIENCIAS and Cementos ARGOS S.A.S. who supports the project. To the Biomineralogy Laboratory (National University of Colombia) where the investigation was carried out.
References
1. H. Calkins. ''The chemical forms of sulfur in coal: a review''. Fuel. Vol. 73. 1994. pp. 475-484. [ Links ]
2. A. Morán, A. Áller, J. Cara, O. Martinez, E. Encinas, E. Gómez. ''Microbiological desulfurization of column- packed coal''. Fuel Processing Technology. Vol. 52. 1997. pp. 155-164. [ Links ]
3. A. Áller, O. Martínez, J. de Linaje, R. Méndez, A. Morán. ''Biodesulphurization of coal by microorganisms isolated from the coal itself''. Fuel Processing Technology. Vol. 69. 2001. pp. 45-57. [ Links ]
4. T. Bozdemir, T Durusoy, E. Erincin, Y. Yürüm. ''Biodesulfurization of Turkish lignites''. Fuel. Vol. 75. 1996. pp. 1596-1600. [ Links ]
5. A. Juszczak, F. Domka, M. Kozlowsky, H. Wachowska. ''Microbial desulfurization of coal with Thiobacillus ferrooxidans bacteria''. Fuel. Vol. 75. 1995. pp. 725-728. [ Links ]
6. C. Eligwe. ''Microbial desulphurization of coal''. Fuel. Vol. 67. 1988. pp. 451-458. [ Links ]
7. J. Klein. ''Technological and economic aspects of coal biodesulfurization''. Biodegradation. Vol. 9. 1998. pp. 293-300. [ Links ]
8. J. Cara, A. Morán, T. Carballo, F. Rozada, A. Áller. ''The biodesulphurization of a semianthracite coal in a packed-bed system''. Fuel. Vol. 82. 2003. pp. 2065-2068. [ Links ]
9. J. Cara, M. Carballo, A. Morán, D. Bonilla, O. Escolano, F. García. ''Biodesulphurization of high sulphur coal by heap leaching''. Fuel. Vol. 84. 2005. pp. 1905-1910. [ Links ]
10. B. Kodali, B. Rao, L. Narasu, R. Pogakub. ''Effect of biochemical reactions in enhancement of rate of leaching''. Chemical Engineering Science. Vol. 59. 2004. pp. 5069-5073. [ Links ]
11. J. Petersen, D. Dixon. ''Competitive bioleaching of pyrite and chalcopyrite''. Hydrometallurgy. Vol. 83. 2006. pp. 40-49. [ Links ]
12. G. Rossi. ''Biodepyritization of coal: achievements and problems''. Fuel. Vol. 72. 1992. pp. 1581-1592. [ Links ]
13. H. Ryu, Y. Chang, S. Kim. ''Microbial coal desulfurization in an airlift bioreactor by sulfur- oxidizing bacterium Thiobacillus ferrooxidans''. Fuel Processing Technology. Vol. 36. 1993. pp. 267-275. [ Links ]
14. A. Malik, M. Dastidara, P. Roychoudhuryb. ''Biodesulphurization of coal: effect of pulse feeding and leachate recycle''. Enzyme and Microbial Technology. Vol. 28. 2001. pp. 49-56. [ Links ]
15. J. Cara, M. Vargas, A. Morán, E. Gómez, O. Martínez, F. García. ''Biodesulphurization of a coal by packed- column leaching. Simultaneous thermogravimetric and mass spectrometric analyses''. Fuel. Vol. 85. 2006. pp. 1756-1762. [ Links ]
16. G. Caicedo, M. Márquez. ''Mecanismo de selección de consorcios bacterianos compatibles con A. ferrooxidans y A. thiooxidans en procesos de biodesulfurización de carbón''. Revista Facultad de Ingeniería Universidad de Antioquia. N° 52. 2010. pp. 88-94. [ Links ]
17. W. Sand, T. Gehrke. ''Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(III) ions and acidophilic bacteria''. Research in Microbiology. Vol. 157. 2006. pp. 49-56. [ Links ]
18. M. Nematí, S. Harrison, G. Handsford, C. Webb. ''Biological oxidation of ferrous sulphate by Thiobacillus ferrooxidans: a review on the kinetic aspects''. Biochemical Engineering Journal. Vol. I. 1998. pp. 171-190. [ Links ]
19. I. Cardona, M. Márquez. ''Biodesulfurization of two Colombian coals with native microorganisms''. Fuel Processing Technology. Vol. 90. 2009. pp. 1099-1106. [ Links ]
20. C. Acharya, R. Kar, L. Sukla. ''Bacterial removal of sulphur from three diferent coals''. Fuel. Vol. 80. 2001. pp. 2207-2216. [ Links ]
21. G. Caicedo, M. Márquez, C. Moreno. ''Influencia de la concentración de hierro y pH iniciales en un proceso de biodesulfurización de carbón - ensayos a nivel de laboratorio''. Revista Colombiana de Biotecnología. Vol. 13. 2011. pp.199-209. [ Links ]
22. M. Ossa, M. Márquez. ''Biooxidación de sulfuros mediante cepas nativas de acidófilos compatibles con Acidithiobacillus ferrooxidans y thiooxidans, mina de oro el Zancudo, (Titiribí, Colombia)''. Revista Colombiana de Biotecnología. Vol. 7. 2005. pp. 55-66. [ Links ]
23. O. García, J. Bigham, O. Tuovinen. ''Oxidation of isochemical FeS2 (marcasite-pyrite) by Acidithiobacillus thiooxidans and Acidithiobacillus ferrooxidans''. Minerals Engineering. Vol 20. 2007. pp. 98-101. [ Links ]
24. I. Suzuki, D. Lee, B. Mackay, L. Harahuc, J.K. Oh. ''Effect of various ions, pH, and osmotic pressure on oxidation of elemental sulfur by Thiobacillus thiooxidans''. Applied and Environmental Microbiology. Vol. 65. 1999. pp. 5163-5168. [ Links ]
25. C. Gahan, J. Sundkvist, A. Sandstrom. ''A study on the toxic effects of chloride on the biooxidation efficiency of pyrite''. Journal of Hazardous Materials. Vol. 172. 2009. pp. 1273-1281. [ Links ]