Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230
Rev.fac.ing.univ. Antioquia no.75 Medellín Apr./June 2015
https://doi.org/10.17533/udea.redin.n75a04
ARTÍCULO ORIGINAL
DOI: 10.17533/udea.redin.n75a04
Chitosan/hydroxyapatite scaffolds for tissue engineering manufacturing method effect comparison
Comparación del efecto del método de fabricación en plataformas de quitosano/hidroxiapatita para ingeniería de tejidos
Diana Marcela Escobar-Sierra1*, Johan Martins2, Claudia Patricia Ossa-Orozco1
1 Grupo de Investigación en Biomateriales (BIOMAT), Programa de Bioingeniería, Facultad de Ingeniería, Universidad de Antioquia. Calle 70 N.° 52-21. Medellín, Colombia.
2 Franche-Comté Higher Institute of Engineering, University of Franche-Comté. 23 Rue Alain Savary TEMIS, 25000. Besançon, France.
* Corresponding author: Diana Marcela Escobar Sierra, e-mail: marcela.escobar@udea.edu.co
(Received October 30, 2014; accepted April 14, 2015)
Abstract
The regeneration of bone is one of the main challenges of modern medicine because many diseases, including trauma and tumor, can cause bone defects. Tissue engineering (TE) is a promising approach to cure these bone diseases because it allows the reconstruction of tissue by colonization and proliferation of healthy cells in an artificial extracellular matrix (scaffolds). The aim of this project was to prepare chitosan/hydroxyapatite CH/HA scaffolds, using various ratios and two different methods: powder hydroxyapatite (commercial) and in situ hydroxyapatite, and then compare their properties. The morphology, chemical composition and mechanical properties were evaluated by Scanning Electron Microscopy (SEM), X-ray diffraction (XRD), Raman spectroscopy, and compression tests. The scaffolds obtained showed an interconnected porous structure. The scaffolds with chitosan and hydroxyapatite manufacturing by in situ protocol, have better applications in tissue engineering, because they have a better morphology and allow the cell growth. These scaffolds are suitable for tissue engineering.
Keywords: bone, chitosan, hydroxyapatite in situ, hydroxyapatite in powder, scaffolds chitosan/ hydroxyapatite, tissue engineering
Resumen
La regeneración del hueso es uno de los principales retos de la medicina moderna debido a que muchas enfermedades, incluyendo los traumas y los tumores, pueden causar defectos óseos. La ingeniería de tejidos (IT) es un área de investigación muy prometedora para curar estas enfermedades de los huesos, ya que permite la reconstrucción de los tejidos basados en la colonización y proliferación de las células sanas, provenientes del mismo paciente, en una matriz extracelular artificial denominada plataformas o andamios. El objetivo de este proyecto fue fabricar plataformas de quitosano/hidroxiapatita CH/HA, utilizando diversas relaciones y dos métodos diferentes para la obtención de la hidroxiapatita, en polvo (comercial) e in situ, para luego comparar sus propiedades. La morfología, la composición química y las propiedades mecánicas se evaluaron por microscopía electrónica de barrido (SEM), difracción de rayos X (XRD), espectroscopia Raman y ensayos de compresión. Los andamios obtenidos presentaron una estructura porosa interconectada. Las plataformas con mejores propiedades para ingeniería de tejidos fueron las fabricadas con quitosano e hidroxiapatita in situ, debido a que su morfología era más óptima para permitir el crecimiento celular. Estos andamios cumplen los requisitos de la ingeniería de tejidos.
Palabras clave: hueso, quitosano, hidroxiapatita in situ, hidroxiapatita en polvo, plataformas quitosano/hidroxiapatita, ingeniería de tejidos
Introduction
The regeneration of bone is one of the major difficulties in clinical surgery because many conditions including trauma, tumor, and bone diseases -such as osteitis and osteomyelitis- can cause bone defects. To restore the normal structure and function of bone, many solutions have been used in therapy and research, including autografts, allografts, xenografts, and other artificial substitutes. Recently, three-dimensional porous scaffolds loaded with specific living cells have been researched in order to regenerate tissue in a natural way [1, 2].
Hydroxyapatite (HA), Ca10(PO4)6(OH)2, is the principal inorganic component of bone; this has been extensively used for biomedical implant applications and bone regeneration. It has a similar chemical composition and structure to the mineral component of natural bone and has showed high biocompatibility, osteoconductivity, and bone bonding ability [1, 3]. Since the natural bone is a composite mainly consisting of collagen and HA, many efforts have been made to modify HA with polymers, such as collagen, gelatin, chitosan, chitin, and polylactic acid (PLA), which have been used to fix the bone. Among these polymers, the biopolymers have received much attention in the field of medical applications, due to their excellent biocompatibility and biodegradability [3-5]. The clinical success of the scaffold for bone regeneration could be associated to combinations of inorganic and organic biomaterials. Due to this fact, the field of bone tissue regeneration has already made progress on researchs for hybrid biomaterials [6].
Chitin is the second most abundant natural polysaccharide (after cellulose). Chitosan (CH) is a derivative of chitin extracted from natural sources, such as the crustacean shells, the exoskeleton of insects, and fungi, obtained by chitin deacetylation. Chitosan is a promising material for medical applications due to its antibacterial properties, low toxicity, biodegradability, biocompatibility with human tissues, and the ability to facilitate regenerative processes in healing wounds. The ability of chitosan to support cell attachment and proliferation is attributed to its chemical properties. The chitosan is structurally similar to glycosaminoglycan, the major component of the extracellular matrix of bone. Other advantages of chitosan scaffolds for bone tissue engineering include the formation of highly porous scaffolds with interconnected pores, osteoconductivity, and ability to enhance bone formation both in vitro and in vivo [7].
Composites comprising calcium phosphates and natural biopolymers as chitosan are widely used as biomaterials for bone tissue engineering. Therefore, a composite biomaterial of HA and chitosan is expected to show increased osteoconductivity and biodegradation together with sufficient mechanical strength for orthopedic use. In recent years, the interest in biomaterials such as hydroxyapatite and chitosan has increased significantly, evidenced by the significant growth in the number of scientific articles reporting their characterization and evaluation [8].
The hydroxyapatite/chitosan composites obtained are characterized by different physicochemical methods to test their potential as biomaterials; and a series of biocompatibility tests using cell cultures have been performed, confirming biocompatibility of these composites. Several authors [9-12] have carried out different tests showing these properties.
The aim of this study was to prepare chitosan/hydroxyapatite scaffolds with different proportions of hydroxyapatite and chitosan, using two different methods proposed by [13]. One with hydroxyapatite in situ and the other with hydroxyapatite in powder previously prepared, coupled with chitosan. The microstructure was evaluated by spectroscopy, microscopy techniques, and compression tests to observe the best method and the best ratio of chitosan/hydroxyapatite to obtain scaffolds with better properties to be used in tissue engineering.
Materials and methods
This study presents a simple but efficient method to prepare CH/HA composite scaffolds with a porous structure. The novel technique involved extraction of precursors, co-precipitation, molding, and consequent freeze-drying.
Chitosan extraction from crustacean shells
The protocol used was based on the protocol proposed by [14], the protocol consists of four steps.
Pre-wash with water: The crab shells were washed several times with hot water to remove residues, lipids, and other contaminants, and dried at room temperature overnight. Later, the shells were fragmented in small pieces with a rotor beater mill, (Retsch S1000) with zirconium oxide balls, for 15 minutes, and finally filtered through 106 µm mesh sieves.
Deproteinization: The powder obtained was treated in a 3.5% NaOH solution at 90°C for 120 minutes, in a ratio of 10% w/v, with constant stirring. Then, several washings were made to neutralize the solution until obtaining distilled water pH, and finally the powder was dried in an oven at 60°C for 6 hours.
Demineralization: The powder obtained from the previous step was treated in 4M HCl solution at room temperature for 90 minutes in a ratio of 20% w/v with constant stirring. Then, several washings were made to neutralize before mentioned solution until obtaining water pH; the material was dried in an oven at 60°C for 6 hours.
Deacetylation: The demineralized powder was treated in a solution of 50% NaOH at 90°C for 180 minutes in a ratio of 10% w/v, with constant stirring. Then, several washings were made to neutralize the solution until obtaining distilled water pH. Then, the powder was centrifuged and dried in an oven at 60°C for 6 hours.
Characterization of raw materials
To manufacture the CH/HA scaffolds, commercial hydroxyapatite powder acquired in Strem Chemicals with an average particle size of 12.9 μm was used. Chitosan obtained was evaluated by X-ray Diffraction (XRD), which provided information of the crystal structure, chemical composition, and physical properties of the sample. This analysis was performed using a Rigaku Miniflex X-ray powder diffractometer, equipped with a Cu source with λ = 1.5818Å at an angle of 2θ and a range of 0° to 60°, with scan rate at 2°/min.
Production of chitosan/hydroxyapatite scaffolds
The CH/HA composite scaffolds were prepared by two different methods and a subsequent freeze-drying process to obtain an adequate morphology and pore size.
Production of scaffolds of chitosan/hydroxyapatite in situ
A solution of chitosan was prepared with a concentration of 2% w/v, dissolving chitosan powder obtained in acetic acid solution at 1% v/v. Then, a solution 0.5M of Ca(NO3)2 was added in the chitosan solution under constant stirring for 30 min, and finally, a solution 0.3M of NH4H2PO4 was added drop by drop as precursors to prepare the hydroxyapatite in situ, keeping the Ca/P ratio equal to 1.67.
The total solution obtained was mixed thoroughly for 2 hours until fully homogenized, and, finally, the solution was put into containers and was frozen 8 hours at -80 °C and lyophilizing for 24 hours in a Labconco freeze dryer. Scaffolds were prepared in chitosan/hydroxyapatite composition (weigh ratio) 70/30, 50/50 and 30/70; they were denominated CH/HAis. Four samples for condition were prepared.
Production of scaffolds of chitosan/hydroxyapatite in powder
A solution of chitosan 2% (w/v) was prepared by dissolving chitosan powder in a solution of acetic acid at 1% (v/v), as the first protocol. Composite scaffolds were synthesized with different ratios 70/30, 50/50 and 30/70; they were called CH/HAip. HA powder was dispersed in deionized water for 2 hours. Subsequently, the suspension was added drop by drop to the chitosan solution, while the solution was being agitated. Next, the CH/HAip suspension was vigorously mixed using a magnetic stirrer for 2 hours to obtain a homogenous mixture, and then was transferred to containers, frozen at -80 °C for 8 hours and lyophilized for 24 hours. Four samples for condition were manufactured.
Characterization of chitosan/hydroxyapatite scaffolds
The composites obtained in such manner were characterized by different physicochemical methods to test their potential as biomaterials. The characteristics of raw HA powder were examined with X-ray Fluorescence (XRF) using a semiquantitative chemical analysis in spectrometer XRF Thermo brand, model Optim'x. The morphological characterization of the scaffold structure was carried out with Scanning Electron Microscopy (SEM) using a JEOL, model JSM 6490 LV equipment, and the functional groups were obtained by Raman Spectroscopy using a Dilor, model LabraM2, over a range of 500 to 3500 cm−1. Compression tests were carried out in a Universal Tests Equipment (Digimess). The tests were conducted in triplicate and performed under the following parameters: speed of deformation: 5mm/min, maximum deformation: 75%, maximum load: 500N. Assays compression stregth of all scaffolds were carried out, to determine the mechanical resistance of scaffolds with a different ratio CH/HA. However, only the results for 70/30 CH/HA was reported, because the test for 30/70 and 50/50 CH/HA did not produce expected results due to the samples were wet yet, consequently results could not be correct.
Results and discussion
Characterization of raw materials
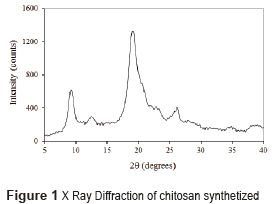
The diffractogram of chitosan obtained from the crab shells is shown in Figure 1. In the literature, chitosan is characterized by the presence of two peaks by X-ray diffraction: a peak at 2θ=9.5° and another at 2θ=19.5° [15]. In this research, these two characteristic peaks were found, and also two peaks of lower intensity: a peak at 2θ=12° and another at 2θ=27° showing some impurities in the sample. Impurities could occur in many phases of manipulation. Consequently, the chitosan obtained was sufficiently pure and semi crystalline to be used in this experiment.
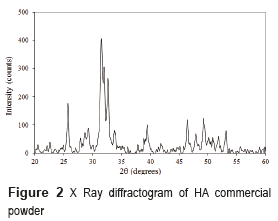
The hydroxyapatite commercial powder was characterized by XRD and its diffractogram is shown in Figure 2. The HA powder diffractogram presents peaks characteristic of the HA, located at 2θ = 31.7, 32.2 and 33°; it is also possible to observe some lower intensity secondary peaks located at 2θ = 26, 40, 46.5, and 49°, and other less intense located at 2θ = 29 and 53.2°, which corroborate the existence of hydroxyapatite. It was concluded that commercial powder used in the foams manufacture did not present any phases different from HA, and it was highly crystalline.
Characterization of chitosan/hydroxyapatite scaffolds
Morphology
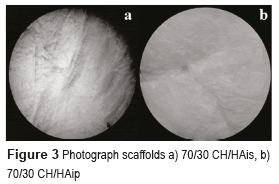
CH/HA composite scaffolds had abundant homogeneous pores with the adequate diameter, which provided appropriate 3-dimensional microstructure to be used in tissue engineering. In the scaffolds obtained with the protocol for CH/HAis, the sample had the best tridimensional structure, the sample with 70/30 CH/HA is visible in Figure 3a. Its shape and color are homogeneous. Its surfaces have a high porosity and stiffness. Similarly, in the scaffolds acquired with the protocol for CH/HAip, samples with a high proportion of chitosan (scaffolds with 70% CH and 30% HA in powder) present the best structure, visible in Figure 3b. They also have high porosity and stiffness. The structure of the scaffolds with 50/50 % and 30/70 % of CH/HAis and CH/HAip are quite similar.
Scaffolds based in hydroxyapatite in powder are whiter than the scaffolds based in hydroxyapatite in situ. The structure is also very different; this presents a planar shape due to the precipitation of the particles of HA in the suspension. Scaffolds have a surface smoother than the previous ones, and present a lower porosity. It is possible to observe many differences among the samples with different proportions of chitosan/hydroxyapatite but each sample of the same proportion presents the same structure, which shows the good reproducibility of these scaffolds. It is observed that the best structures of scaffolds are scaffolds with a high proportion of chitosan (scaffolds with 70% CH and 30% HA) for both protocols. The color also changed, the scaffolds based on hydroxyapatite in powder are whiter than the scaffolds based on hydroxyapatite in situ.
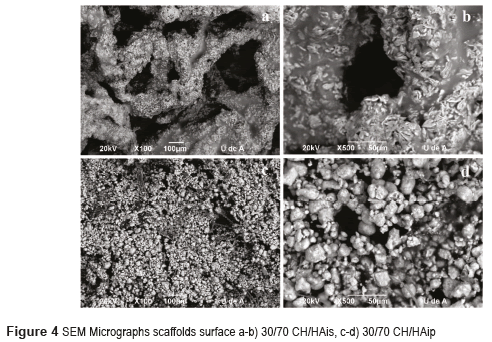
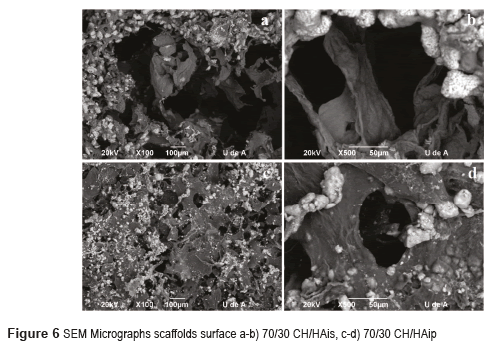
Scaffolds with 30% CH and 70% HA micrographs by Scanning Electron Microscopy SEM are shown in Figures 4 for HA in situ and HA in powder. Figures 5 and 6 show the micrographs for the scaffolds with 50% CH and 50% HA and 70% CH and 30% HA, respectively.
Scaffolds CH/HA in situ show a three-dimensional matrix with a general porosity and a high interconnection among the pores. These morphological characteristics are ideal for applications in tissue engineering because they allow the colonization of cells to assure a good integrity and functionality of the osteochondral construction.
The chitosan and hydroxyapatite were homogenously combined through the in situ synthesis of HA using the co-precipitation method, and the porous structure generated by the lyophilization showed good porosity and some cells could grow in the pores of these 3-D scaffolds. Also, on scaffolds pore walls, the HA particles were inlaid in the chitosan surface like islands in concordance with reported by [11].
Scaffolds for protocol with hydroxyapatite in powder have a three-dimensional matrix with fewer pores. It may be observed that the shape of hydroxyapatite is also different; the hydroxyapatite crystals have a rounded shape.
From the SEM images shown in Figure 4a, it can be observed that CH/HA bilayer scaffolds microarchitecture has a high porosity and opened pore structure, in accordance with [16]. It may be noted that the matrix is composed of chitosan and the surface of the matrix is coated with hydroxyapatite in form of fine crystals (Figure 4a and 4b). A good distribution of hydroxyapatite on the surface of the scaffolds is also observed. Hydroxyapatite particles are crystals that seem to have a growth direction given by the thermal gradient during the freezing process at the same time that by maturing of hydroxyapatite. The homogeneous distribution of HA particles on the scaffold surface can give more contacting areas for bone cells deposition, that is a benefit for the biomineralization, as shown in Figure 4. The homogeneous distribution of HA particles on the scaffold surface can give more contacting areas for bone cells [17]. The Figure 4c and 4d show a dense and opaque structure; these scaffolds have a large amount of hydroxyapatite, because the hydroxyapatite particles are accumulated and cover the entire surface of the scaffolds and also cover the pores in accordance with [4]. This structure does not seem to be interesting for tissue engineering because the cells cannot penetrate the scaffold.
In the CH/HAis scaffolds, when decreasing the amount of hydroxyapatite, it is evident that fewer particles are available, and located evenly on the outer walls of the pores, as can be seen in Figures 5a-b and 6a-b. The pores have a diameter in the range of 50–100 mm. In agreement with [18, 19], the pores of 40-100 mm will allow ingrown blood vessels to facilitate vascularization and bone mineralization. In this case, the scaffolds have pores of these dimensions; consequently, they could be used for bone reconstruction. The presence of small pores less than 20 mm is also important for the protein absorption, ionic solubility, and the attachment of osteoblasts on the scaffolds [20]. This study revealed that CH/HAis scaffolds pores quite resemble a typical spongy 3D structure, with open pores, anisotropic porosity, and pore size ranging from 50-100 μm, as reported by [16, 21]. The surface morphology of the composite scaffold was rough because of the introduction of HA crystals in concordance with [22].
Scaffolds with protocol with hydroxyapatite in powder have a three-dimensional matrix with fewer pores (Figures 5c and 6c). The pores are small between 5-40mm. It may be observed that the shape of hydroxyapatite is also different; the hydroxyapatite crystals have a rounded shape (Figures 5d and 6d). Scaffolds with hydroxyapatite in powder seem to have a denser structure with a lower porosity in comparison with other scaffolds.
The pore size in both kinds of scaffolds decreased with the increasing of HA content. In concordance with [21, 23], it seems that in CH/HAis, once HA nuclei were formed on the surface of chitosan scaffold, could grow and spread out on the surface of scaffold with the increasing HA content.
With these results of SEM, it could be said that scaffolds which have the best structural integrity and porosity are the scaffolds of CH/HAis. They are good candidates for tissue engineering and bone regeneration.
All composite scaffolds were similar in their macroscopic morphology, which indicated that adding the HA in the system did not influence the porous structure; however, the microscopic morphology on pore-wall surfaces was quite different because of different crystals formed. Also, the quantity of the HA particles varied with the various amounts of HA added as powder or in situ, in agreement with [11].
Raman Spectroscopy
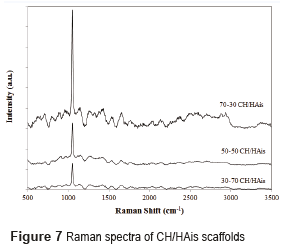
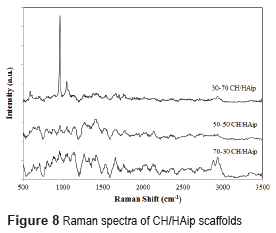
The Raman spectrum for scaffolds with hydroxyapatite in situ and in powder is shown in Figures 7 and 8 respectively.
The analyzes conducted by Raman´s spectroscopy, in scaffolds composed of hydroxyapatite in powder, and of hydroxyapatite in situ, found the presence of hydroxyapatite and chitosan in the prepared scaffolds. The Raman spectrum of chitosan is characterized by a high band at 1070 cm-1, attributed to stretching vibrations υ(O=S=O), a band around 588 cm−1 corresponds to deformation vibrations d(O=S=O), and other bands between 823 cm−1 and 834 cm−1 arises from the stretching vibrations υ(C–O–S) [15]. The Raman spectrum of hydroxyapatite is characterized by a high band at 960 cm-1 assigned to the symmetric νsP–O(H) stretching vibration, whereas the other bands (1036, 1088, 1072 cm−1) are assigned to both δPO–H bending and νasP–OH asymmetric stretching vibrations as reported by [24].
Raman spectrum of scaffolds CH/HAis is characterized by a peak of chitosan at 1070 cm-1, with an intensity difference that reflects the different proportions of chitosan in the samples. The high peak at 960 cm-1 of hydroxyapatite was not found. This is due to the fact that these scaffolds are not crystalline hydroxyapatite compounds previously formed (after a calcination process at high temperatures), but rather with hydroxyapatite precursors to form it in situ.
The Raman spectra of CH/HAip scaffolds are different. The Raman spectrum of 30/70 (Figure 8) shows the characteristic peaks of hydroxyapatite and chitosan at 960 cm-1 and 1070 cm-1 respectively. Furthermore, the peaks have different intensities.
The peak of hydroxyapatite is more intense than the peak of chitosan, what illustrates the difference in proportion between hydroxyapatite (70%) and chitosan (30%). And, in comparison with Raman´s spectra of bone, the composition of the scaffolds of 30% CH/ 70% HA in powder is very similar to the composition of bone [25].
Compression Tests
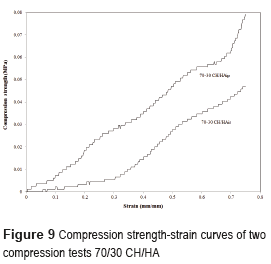
The mechanical performance of the CH/HA composite scaffolds is one of the most important factors determining both their postsurgical process and bone healing capacity. In tissue engineering, the scaffolds should have mechanical properties sufficient for supporting new bone tissue regeneration at the site of implantation and maintaining sufficient integrity for both in vitro and in vivo cells. Figure 9 shows the representative stress–strain curves for two series of 70CH/30HA composite scaffolds, for different protocol, one test with a scaffold of chitosan and hydroxyapatite in situ and the other test with a scaffold of chitosan and hydroxyapatite in powder.
The Figure 9 shows typical stress-strain behaviors for these composite scaffolds tested until the specimens were compressed to approximately 75% of their original height. The initial linear region defines the compressive modulus of the scaffolds. According to the curves, the compression strength is more important to the scaffolds with hydroxyapatite in powder with a maximum stress of 0.08 MPa for a deformation of 75%, while the other has a stress about 0.047 MPa. Two regions in the curve, a plateau due to the plastic collapse and buckling of the elements, and a further region, where the stress increases rapidly due to the effective densification of the foam structure were observed [23]. It is possible to notice that the compressive strength of scaffolds CH/HAip was to be higher, which may be associated to the low porosity of scaffolds respect to CH/HAis and the other calcium phosphate phases present in this scaffold that have compressive strength lower.
The stiffness is greatly improved with the addition of HA. This shows a relationship between the ratio of hydroxyapatite/chitosan and the stiffness of scaffolds [22].
In tissue engineering, the scaffolds should have mechanical properties sufficient for supporting new bone tissue at the site of implantation and maintaining sufficient integrity for both in vitro and in vivo cells. The compressive strength obtained is lower than the one of the bone (2–10 MPa). According to [26], it is possible that the compressive strength of the scaffold had a relation to the composition content, not only to the porosity, because the percentage HA particles could reduce the formation network structure matrix, may be insufficient to bind the higher percentage HA of particles, resulting in poor mechanical properties.
The compressive modulus and the yield strength shown by 70CH/30HAip scaffolds were higher because HA provided mechanical resistance. As can be seen, both the compressive modulus and the yield strength were greatly improved with the addition of HA, in accordance with [27, 28]. The CH/HA scaffold which exhibited excellent elasticity as shown in Figure 9. This means that the brittleness of HA scaffold could be improved by combining with chitosan.
Conclusions
Scaffolds with protocol for chitosan and hydroxyapatite in situ have a three-dimensional matrix with a highly porous microscopic structure and a high interconnection betwen the pores, and this porosity increases with the amount of hydroxyapatite. Meanwhile the scaffolds with chitosan and hydroxyapatite in powder protocol has a three-dimensional matrix with less porosity and smaller pores.
The compression tests show the difference of mechanical strength between the two types of scaffolds. Scaffolds of chitosan and hydroxyapatite in powder have a higher mechanical strength than the scaffolds of chitosan and hydroxyapatite in situ.
Scaffolds with better applications in tissue engineering are the scaffolds with chitosan and hydroxyapatite in situ protocol. Due to their better morphology, they allow the penetration of cells, the transfer of nutrients and oxygen for cell growth. These scaffolds meet the requirements of tissue engineering.
This study has shown that desirable pore structure, mechanical properties, and chemical composition of the composite scaffolds might be achieved through the control of the procedure and the ratio of both hydroxyapatite and chitosan.
Ackowledgements
The authors of this study express their gratitude to Biomaterials Research Group of Bioengineering Program and Polymers and Ceramics Laboratories of Materials Department Universidad of Antioquia.
References
1. S. Mistry, A. Mikos. ''Tissue engineering strategies for bone regeneration''. Adv Biochem Engin/Biotechnol. Vol. 94. 2005. pp. 1-22. [ Links ]
2. P. Costantino, C. Friedman. ''Synthetic bone graft substitutes''. Otolaryngol Clin North Am. Vol. 27. 1994. pp. 1037-1074. [ Links ]
3. S. Jun, E. Lee, T. Jang, H. Kim, J. Jang, Y. Koh. ''Bone morphogenic protein-2 (BMP-2) loaded hybrid coating on porous hydroxyapatite scaffolds for bone tissue engineering''. J Mater Sci Mater Med. Vol. 24. 2013. pp. 773-782. [ Links ]
4. F. Zhao, Y. Yin, W. Lu, J. Chiyan, W. Zhang, J. Zhang, M. Zhang, K. Yao. ''Preparation and histological evaluation of biomimetic three-dimensional hydroxyapatite/chitosan-gelatin network composite scaffolds''. Biomaterials. Vol. 23. 2002. pp. 3227-3234. [ Links ]
5. T. Keane, S. Badylak. ''Biomaterials for tissue engineering applications''. Seminars in Pediatric Surgery. Vol. 23. 2014. pp. 112-118. [ Links ]
6. A. Shrivats, M. McDermott, J. Hollinger. ''Bone tissue engineering: state of the union''. Drug Discovery Today. Vol. 19. 2014. pp. 781-786. [ Links ]
7. M. Vallet. ''Ceramics for medical applications''. J. Chem. Soc., Dalton Trans. Vol. 1. 2001. pp. 97-108. [ Links ]
8. S. Teixeira, M. Rodriguez, P. Pena, A. Aza, S. Aza, M. Ferraz, F. Monteiro. ''Physical characterization of hydroxyapatite porous scaffolds for tissue engineering''. Mater Sci Eng C. Vol. 29. 2009. pp. 1510-1514. [ Links ]
9. B. Chesnutt, A. Viano, Y. Yuan, Y. Yang, T. Guda, M. Appleford, J. Ong, W. Haggard, J. Bumgardner. ''Design and characterization of a novel chitosan/nanocrystalline calcium phosphate composite scaffold for bone regeneration''. J Biomed Mater Res Part A. Vol. 88. 2009. pp. 491-502. [ Links ]
10. S. Danilchenko, O. Kalinkevich, M. Pogorelov, A. Kalinkevich, A. Sklyar, T. Kalinichenko, et al. ''Chitosan–hydroxyapatite composite biomaterials made by a one step co-precipitation method: preparation, characterization and in vivo tests''. J Biol Phys Chem. Vol. 9. 2009. pp. 119-126. [ Links ]
11. L. Kong, Y. Gao, W. Cao, Y. Gong, N. Zhao, X. Zhang. ''Preparation and characterization of nano-hydroxyapatite/chitosan composite scaffolds''. J Biomed Mater Res A. Vol. 75. 2005. pp. 275-282. [ Links ]
12. Z. Li, L. Yubao, Y. Aiping, P. Xuelin, W. Xuejiang, Z. Xiang. ''Preparation and in vitro investigation of chitosan/nano-hydroxyapatite composite used as bone substitute materials''. J Mater Sci Mater Med. Vol. 16. 2005. pp. 213-219. [ Links ]
13. C. Peniche, Y. Solís, N. Davidenko, R. García. ''Chitosan/hydroxyapatite-based composites''. Biotecnol Apl. Vol. 27. 2010. pp. 202-210. [ Links ]
14. D. Escobar, C. Ossa, M. Quintana, W. Ospina. ''Optimización de un protocolo de extracción de quitina y quitosano desde caparazones de crustáceos''. Scientia et Technica. Vol. 18. 2013. pp. 260-266. [ Links ]
15. M. Yen, J. Yang, J. Mau. ''Physicochemical characterization of chitin and chitosan from crab shells''. Carbohydr Polym. Vol. 75. 2009. pp. 15-21. [ Links ]
16. J. Oliveira, M. Rodrigues, S. Silva, P. Malafaya, M. Gomes, C. Viegas, I. Dias, J. Azevedo, J. Mano, R. Reis. ''Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: Scaffold design and its performance when seeded with goat bone marrow stromal cells''. Biomaterials. Vol. 27. 2006. pp. 6123-6137. [ Links ]
17. J. Chen, K. Nan, S. Yin, Y. Wang, T. Wu, Q. Zhang . ''Characterization and biocompatibility of nanohybrid scaffold prepared via in situ crystallization of hydroxyapatite in chitosan matrix. Colloids Surf B Biointerfaces. Vol. 81. 2010. pp. 640-647. [ Links ]
18. J. Klawitter, S. Hulbert. ''Application of porous ceramics for the attachment of load bearing internal orthopaedic applications''. J Biomed Mater Res Symp. Vol. 5. 1971. pp. 161-229. [ Links ]
19. T. Wu, K. Nan, J. Chen, D. Jin, S. Jiang, P. Zhao, J. Xu, H. Du, X. Zhang, J. Li, G. Pei. ''A new bone repair scaffold combined with chitosan/ hydroxyapatite and sustained releasing icariin''. Chinese Sci Bull. Vol. 54. 2009. pp. 2953-2961. [ Links ]
20. K. Zhang, D. Peschel, J. Helm, T. Groth, S. Fischer. ''FT Raman investigation of novel chitosan sulfates exhibiting osteogenic capacity''. Carbohydr Polym. Vol. 83. 2011. pp. 60-65. [ Links ]
21. S. Teng, E. Lee, P. Wang, S. Jun, C. Han, H. Kim. ''Functionally Gradient Chitosan/Hydroxyapatite Composite Scaffolds for Controlled Drug Release''. J Biomed Mater Res Part B: Appl Biomater. Vol. 90. 2009. pp. 275-282. [ Links ]
22. K. Im, J. Park, K. Kim, K. Kim, S. Choi, C. Kim, Y. Lee. ''Organic-Inorganic hybrids of hydroxyapatite with chitosan''. Key Eng. Mater. Vol. 284-286. 2005. pp. 729-732. [ Links ]
23. H. Jin, C. Lee, W. Lee, J. Lee, H. Park, S. Yoon. ''In-situ formation of the hydroxyapatite/chitosan-alginate composite scaffolds''. Mater Lett. Vol. 62. 2008. pp. 1630-1633. [ Links ]
24. I. Cukrowski, L. Popovic, W. Barnard, S. Paul, P. Rooyen, D. Liles. ''Modeling and spectroscopic studies of bisphosphonate–bone interactions. The Raman, NMR and crystallographic investigations of Ca-HEDP complexes''. Bone. Vol. 41. 2007. pp. 668-678. [ Links ]
25. C. Tchanque, B. Gong, B. Poushanchi, A. Donneys, D. Sarhaddi, K. Gallagher, S. Deshpande, S. Goldstein, M. Morris, S. Buchman. ''Raman spectroscopy demonstrates Amifostine induced preservation of bone mineralization patterns in the irradiated murine mandible''. Bone. Vol. 52. 2013. pp. 712-717. [ Links ]
26. L. Jiang, Y. Li, X. Wang, L. Zhang, J. Wen, M. Gong. ''Preparation and properties of nano-hydroxyapatite/chitosan/carboxymethyl cellulose composite scaffold''. Carbohydr Polym. Vol. 74. 2008. pp. 680-684. [ Links ]
27. S. Teng, E. Lee, B. Yoon, D. Shin, H. Kim, J. Oh. ''Chitosan/nanohydroxyapatite composite membranes via dynamic filtration for guided bone regeneration''. J Biomed Mater Res Part A. Vol. 88. 2009. pp. 569-580. [ Links ]
28. J. Jancár, A. Slovíková, E. Amler, P. Krupa, H. Kecová, L. Plánka, P. Gál, A. Necas. ''Mechanical response of porous scaffolds for cartilage engineering''. Physiol. Res. Vol. 56. 2007. pp. 17-25. [ Links ]