Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230
Rev.fac.ing.univ. Antioquia no.75 Medellín Apr./June 2015
https://doi.org/10.17533/udea.redin.n75a05
ARTÍCULO ORIGINAL
DOI: 10.17533/udea.redin.n75a05
Effect of the functionalization of silica nanoparticles as a reinforcing agent on dental composite materials
Efecto de la funcionalización de nanopartículas de sílica como agente de refuerzo en materiales dentales compuestos
Henry Alberto Rodríguez-Quirós1*, Herley Fernando Casanova-Yepes2
1 Grupo de Investigación New Stetic (GINEWS), New Stetic S.A. Carrera 53 N.° 50-09. Guarne, Colombia.
2 Grupo de Coloides, Instituto de Química, Universidad de Antioquia. Calle 67 N.° 53-108. Medellín, Antioquia.
* Corresponding author: Henry Alberto Rodríguez Quirós, e-mail: hrodriguez@newstetic.com
(Received September 08, 2014; accepted April 22, 2015)
Abstract
The present study evaluated the effect of silica nanoparticle aggregation state on the reflectance and crystallinity of dental composite materials. Two types of silica nanoparticles (ca. 10 nm): Aerosil 200® non-funcionalized and Aerosil DT4® funcionalized with 3-methacryloxypropyltrimethoxysilane. Nanoparticles were dispersed in a monomer mix composed by Urethane Dimethacrylate (UDMA) and Ethylene glycol Dimethacrylate (EGDMA) in a 80:20 mass ratio. The particle size of silica and their aggregation state were determined using scanning electron microscopy (SEM) and transmission electron microscopy (TEM), showing that the Aerosil DT4® has dense aggregates with sizes higher than 1 µm; on the other hand the Aerosil 200® showed a particle gel-like structure. The functionalization degree of the Aerosil DT4® was determined by thermogravimetric analysis (TGA), obtaining a value of 7.57% w/w. The composite materials were evaluated by Differencial Scanning Calorimerty (DSC) to determine their crystallinity. The composite material reinforced by Aerosil DT4® showed lower cristallinity than the system with Aerosil 200® due to higher interaction of the polymeric matrix with the funcionalized surface of the Aerosil DT4®. The effect of the aggregation state of silica nanoparticles on the optical properties of the composite material was determined by reflectance analysis. The Aerosil 200® sample showed a lower degree of nanoparticle aggregation and higher reflectance than the system with Aerosil DT4®. The functionalization of the Aerosil DT4® induced nanoparticle aggregation diminishing the optical properties of the composite material.
Keywords: composite material, functionalization, silica, UDMA, EGDMA, particle size, dental materials
Resumen
El presente estudio evaluó el efecto del estado de agregación de nanopartículas de sílica en la reflectancia y cristalinidad de materiales compuestos de uso dental. Se emplearon dos tipos de sílica nanométrica (ca. 10 nm): Aerosil 200® no funcionalizado y Aerosil DT4® funcionalizado con 3-metacriloxipropiltrimetoxisilano. Las nanopartículas de sílica fueron dispersas en una mezcla de monómeros de Uretano Dimetilacrilato (UDMA) y Etilenglicol Dimetacrilato (EGDMA) en una relación 80:20 en masa. El tamaño de partícula de la silica y su estado de agregación fue determinado mediante microscopía electrónica de barrido (SEM) y microscopía electrónica de transmisión (TEM), mostrando que el Aerosil DT4® presentó agregados densos de tamaño superior a 1 µm; en tanto el Aerosil 200® presentó una estructura agregada tipo gel de partículas. El grado de funcionalización del Aerosil DT4® fue determinado mediante análisis termogravimétrico (TGA), obteniendo un valor de 7.57% w/w. Los materiales compuestos fueron evaluados mediante calorimetría diferencial de barrido (DSC) para determinar su cristalidad. El material compuesto reforzado con Aerosil DT4® presentó una menor cristalinidad que el sistema con Aerosil 200®, debido a la mayor interacción de la matriz polimérica con la superficie funcionalizada del Aerosil DT4®. El efecto de la agregación de las nanopartículas de silica en las propiedades ópticas del material compuesto fue determinado mediante análisis de reflectancia. La muestra de Aerosil 200® presentó un menor estado de agregación de las nanopartículas y mayor reflectancia que el sistema con Aerosil DT4®. La funcionalización de la superficie del Aerosil DT4® propició la aglomeración de las nanopartículas deteriorando las propiedades ópticas del material compuesto.
Palabras clave: material compuesto, funcionalización, sílica, udma, egdma, tamaño de partícula, materiales dentales
Introduction
A wide range of materials have been used for teeth restoration. In 1816 Auguste Taveau developed what is considered the first tooth filler [1]. The search for more aesthetic materials ended up into the use of silicates during the first half of the 20th century. Despite having a similar tonality to natural teeth, they were easily worn down. Acrylic resins replaced silicates at the end of the 1940s and beginning of the 50s due to their similar appearance to teeth, insolubility in oral fluids, easy use and low cost. However, acrylic resins are also easily worn down. This situation was improved partly by using filling materials such as quartz, Nevertheless, these composite materials did not have much success due to the lack of adherence between the polymer and filler particles. The most important advance in dental filling materials came in 1962 when Ray L. Bowen developed bisphenol-A-glycidyl methacrylate (Bis-GMA) and the bonding agent vinyl Trichlorosilane, which was reliable for binding particles to the polymeric matrix [1].
Currently, dental composite materials are employed to restore front teeth and to treat small to medium sized lesions in back teeth. For large restorations, ceramic materials are used. Here, a composite material is a mixture that consists of an organic matrix and inorganic fillers that act as a reinforcing material. Usually, the organic matrix is based on methacrylates, specially cross-linked dimethacrylates such as 2,2-bis[4-(2-hydroxy-3-methacryloyloxypropyl)phenyl]propane (Bis-GMA), 1,6-bis-[2-methacryloyloxyethoxycarbonylamino]-2,4,4-trimethylhexane (UDMA), dodecanodiol dimethacrylate (D3MA) and ethylene glycol dimethacrylate (EGDMA), among others [2-4]. The materials commonly used for fillings are silicon dioxide [2, 5], barium or strontium-containing silicate glass powder [2, 6], quartz [1, 2], and titanium and zirconium oxides [2, 7, 8]. To achieve a chemical bond between the filler particles and the dental material, organic silanes such as 3- Methacryloyloxy propyltrimethoxysilane are used [2].
To improve composite dental materials used for teeth restoration, research has been carried out on each of the three components of dental materials (the polymer matrix, the polymer-filler bonding agents, and the filler material). In the case of the organic matrix, research has been focused on new monomers that decrease the contraction during polymerization because this is one of the main problems using polymeric resins as restorative material. Currently, new monomers are under investigation looking for open ring polymerization, such as the spiro orthocarbonates and the vinylcyclopropanes [2, 9]. Likewise, monomers like dendritic and branched monomers [2] have been explored. Monomers that improve the stability of the material in the mouth, once it has been polymerized, have also been investigated, such as modified Bis-GMA molecules (e.g. CH3 Bis-GMA and CF3 Bis-GMA) [10].
Organosilanes are the most commonly used bonding agents [11, 12]. Studies have focused on the effects of structural modifications, in order to improve the stability between the bonding agent and the filler material in a damp environment [13, 14]. Additionally, research has been carried out to modify the length of the organic chain of the bonding agent [11, 15] by looking at groups that increase the hydrophobicity; such as fluorides [16] and phenyls [17], among others.
In terms of filler materials, the studies have focused on obtaining a good dispersion and formation of organized structures to improve the interaction between the particles and the polymer matrix [18]. They have also focused on the effect of particle size and particle concentration in the performance of restored materials [19, 20]. Another important area of investigation is the use of nanoparticles to improve composite material performance, due to their large surface area. These investigations have focused on the effect of nanoparticles functionalization on the mechanical and optical properties and physiochemical stability of dental composite materials [7, 21, 22]. Similar investigations have been made with silica nanoparticles as reinforcing agents in thermoplastic materials [23] and elastomeric matrixes [24].
This study aims to determine the effect of functionalization of silica nanoparticle surface on their aggregation state, which could induce changes in the optical and crystallinity properties of dental composite materials.
Experimental
Materials
Two silicas were employed as reinforcing materials: a non-functionalized hydrophilic silica named Aerosil 200®, from Evonik-Degussa, with an average particle size of 12 nm and a specific surface area of 200 ± 25 m2/g [5]; and a 3-methacryloxypropyltrimethoxysilane functionalized silica, named Aerosil DT4, from Evonik-Degussa with an average particle size between 10 and 20 nm and a specific surface area of 160 ± 25 m2/g and a carbon content of 4 to 6% according to the technical specifications from the provider. The monomers employed were Urethane Dimethacrylate (UDMA) and Etilenglicol Dimethacrylate (EGDMA), supplied by Esstech Inc.
Silica characterization
TGA analysis was carried out on a Q500 instrument (TA instruments), using a temperature interval of 25 to 900°C and a heating velocity of 10°C/min, under a nitrogen atmosphere. Analyses of particle size and morphology were carried out on a scanning electron microscope (SEM) JSM-590LV (JEOL,) and a transmission electron microscope (TEM) JEM 1011 JEOL.
Preparation of the composite material
The composite material (10% w/w Aerosil) was prepared by manual dispersion of the reinforcing material into a mixture of UDMA and EGDMA with a weight ratio of 80:20, respectively. The dispersion was made using a metallic spatula until aggregates were not visually observed. Benzoyl peroxide (0.5% w/w), was used as an initiator. The mixture was polymerized under the following conditions: 15 minutes at a temperature of 80°C and a pressure of 13.1 ± 0.7 MPa and 5 minutes at a temperature of 110°C and a pressure of 13.1 ± 0.7 MPa.
Characterization of the composite material
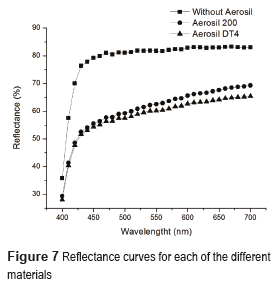
The composite material was characterized by differential scanning calorimetry (DSC), employing a DSC 2920 instrument (TA instruments). These analyses were carried between out 25 to 300°C, at a heating rate of 20°C/min and in a nitrogen atmosphere, at a flow of 50 ml per minute. An indirect qualitative determination of particle size was carried out after polymerization by measuring the percentage of reflected light within the wavelength range of visible light (between 400 and 700 nm). In order to achieve this, reflectance measurements were taken employing a CD6836 spectrometer (BYK Gardner) and a white colored base as a sample in each of the measurements. The degree of translucence was determined by comparing the measurements of the white base with those obtained from each of the samples. The bigger the absolute differences between the reflectance readings of the white base and each sample, the lower the translucence, which indicates the presence of bigger particles size. This is because particles with sizes smaller than the wavelength associated to the incident visible light do not produce scattering and can be used for the manufacturing of transparent materials [25]. The bigger the particle size, the more light is dispersed diminishing the translucence of the material.
Results and discussion
Characterization of the silicas
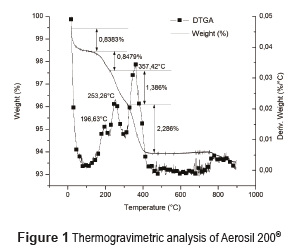
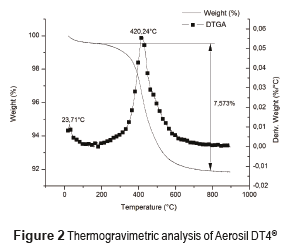
Figure 1 shows the TGA analysis of Aerosil 200® and Figure 2 shows the corresponding analysis for Aerosil DT4®. Aerosil 200® shows a water loss of 1.69% w/w at temperatures below 200°C [26], whereas Aerosil DT4® had a lost of 0.46%. This is because Aerosil 200® is a hydrophilic substance with Si-OH groups at the surface. In contrast, Aerosil DT4® is a hydrophobic substance due to the silanization processes. The Aerosil 200® initial loss in mass occurs in two steps, at temperature below 100 °C, the water probably is physically adsorbed, while at temperatures between 100 °C and 200 °C the water, probably is chemically adsorbed through hydrogen bridges with the Si-OH at the surface, and the desorption process is more difficult. In Aerosil 200®, between 200°C and 400°C, there is a loss in mass associated with the condensation of the silanol groups and the formation of siloxane bonds at the surface. This process occurs in two steps, the first one between 200°C and 300°C corresponds to the silanol condensation of groups at the surface that were previously bonded by hydrogen bridges, and require less energy for their condensation; the second step between 300°C and 400°C occurs between silanol groups that do not have any type of interaction and require greater energy for their condensation [27]. Finally, the Aerosil 200® shows a thermal event at a temperature close to 800°C, corresponding to the formation of siloxane bonds. At temperatures close to 800–900°C, the silica surface becomes free of geminal OH groups and the concentration of siloxane bridges increases considerably. Therefore, the silica surface becomes covered by Si-O-Si groups [28]. The Aerosil DT4 presents only one thermal event between 200 and 800°C, due to the surface decomposition of the silanizing agent.
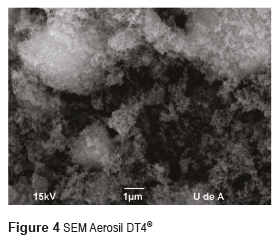
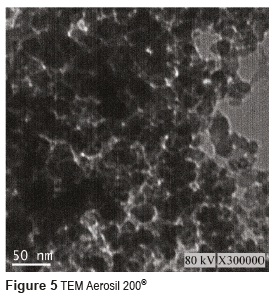
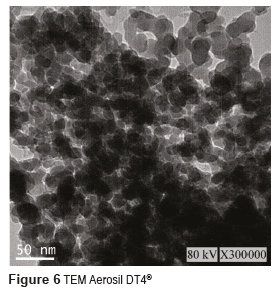
Figures 3 and 4 show the SEM micrographs of the two Aerosil reinforcing materials, whereas Figures 5 and 6 present their TEM. From these figures, it can be seen that the size of the individual particles is less than 50 nm for both types of Aerosil. The manufacturer of Aerosil 200® states that the particle size starts at 12 nm, and according to TEM micrographs the particle size of Aerosil DT4® is between 10 and 20 nm. Both systems present a certain degree of particle aggregation. These clusters are much bigger in Aerosil DT4®, which may be due to the fact that this material is subjected to both functionalization and drying processes.
Preparation of particle dispersion in the monomer mixture
Functionalized Aerosil DT4® showed a better dispersibility in the monomer mixture than non-functionalized materials. The suspension with the fuctionalized Aerosil DT4® was a viscous liquid, while the non-functionalized material showed a paste-like consistency with a much higher viscosity. The difference in the flow properties between the two systems may be associated with two factors. The first one considers that the non-functionalized particles tend to interact with each other through the OH groups at the surface, generating a gel-like structure. In contrast, the functionalized particles interact better with the monomer due to the presence of the functionalizing groups at the surface. The second factor is related to the larger size of the functionalized particles, which induce a reduction in the suspension viscosity [29].
Characterization of the composite material
Figure 7 shows the reflectance results for the materials under study. The presence of the reinforcing material in both systems decreased the light reflected from the white base, due to the dispersion of light induced by the particles present in the sample [14]. The sample with Aerosil DT4® has the lowest reflectance, because of the presence of big particle clusters, probably generated during the functionalization process. The use of particles in composite materials with sizes lower than 100 nm generate materials with high translucence [30], therefore presence of particles or aggregates with sizes above 100 nm, induced lost in the translucence of the composite material. High transparence is obtained for systems with low particle aggregation, even low differences in transmitted light corresponded to important changes in particle size in the order of 200 to 300 nm [31]. Therefore, the sample with Aerosil DT4 which has the lowest reflectance, probably has the bigger particle size associated.
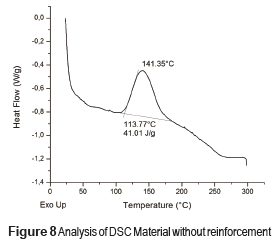
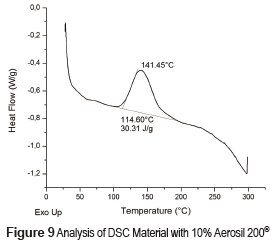
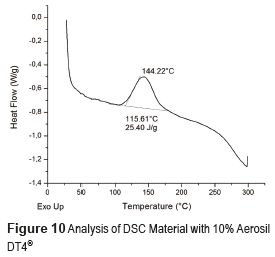
DSC profiles are shown in Figures 8, 9 and 10 that include the crystallization parameters. The maximum crystallization temperatures are similar for both reinforcing materials, although the crystallization temperature is slightly higher for the Aerosil DT4®, probably due to the strong interaction between the polymer molecules and the functionalizing groups at the surface of the latter. This interaction limits the movement of the polymer molecules and increases the crystallization temperature [32]. In contrast, the Aerosil 200® surface has lower interactions with the polymer molecules. The degree of crystallinity of a material is proportional to the absolute value of the enthalpy. Therefore, the degree of crystallization of both composite materials Aerosil 200® and the Aerosil DT4® is lower than the material without reinforcement. Such results suggest that in both cases the reinforcing agent induces a more random structure among the polymer molecules, reducing the crystallinity of the composite material. The effect is bigger in the Aerosil DT4®, where in addition to the influence of the particles themselves, functionalization generates a higher interaction between the polymer and the particles. This is due to the fact that segments of the polymer chains are chemically bonded to the particles, making it even more difficult for them to organize.
Conclusions
The use of silica nanoparticles as reinforcing agents of dental materials has been widely studied, and there are plenty of commercially available materials, which could have significantly differences in terms of their size and surface functionalization. A functionalized silica nanoparticle such as the Aerosil DT4®, available in the market, improved the interaction between the polymer matrix and the nanoparticles, but induced their aggregation, as showed by the SEM micrographs and the reflectance data. The DSC results showed that the presence of particles significantly modifies the crystallinity of the composite material, especially those that are functionalized as they are chemically bounded to the polymeric matrix.
Acknowledgements
This work was carried out thanks to the support from the personnel of the laboratory of CIDEMAT at University of Antioquia and the materials provided by New Stetic S.A.
References
1. K. Anusavice. Phillips ciencia de los materiales dentales. 10th ed. Ed. McGraw-Hill Interamericana Editores, S.A. de C.V. México. 1998. pp. 283-311. [ Links ]
2. N. Mozner, U. Salz. ''Composites for dental restoratives''. W. Shalaby, U. Salz (editors). Polymers for Dental and Orthopedic applications. 1st ed. Ed. Taylor & Francis Group. Boca Raton, USA. 2007. pp. 13-69. [ Links ]
3. C. Pfeifer, Z. Shelton, R. Braga, D. Windmoller, J. Machado, J. Stansbury. ''Characterization of dimethacrylate polymeric networks: A study of the crosslinked structure formed by monomers used in dental composites''. European Polymer Journal. Vol. 47. 2011. pp. 162-170. [ Links ]
4. J. Stansbury. ''Dimethacrylate network formation and polymer property evaluation as determined by the selection of monomers and curing conditions''. Dent. Mat. Vol. 28. 2012. pp. 13-22. [ Links ]
5. M. Atai, A. Pahlavan, N. Moin. ''Nano-porous thermally sintered nano silica as novel fillers for dental composites''. Dent. Mat. Vol. 28. 2012. pp. 133-145. [ Links ]
6. L. Valente, S. Peralta, F. Ogliari, L. Cavalcante, R. Moraes. ''Comparative evaluation of dental composites based on micro-and submicron-sized monomodal glass filler particles''. Dent. Mat. Vol. 29. 2013. pp. 1182-1187. [ Links ]
7. Y. Xia, F. Zhang, H. Xie, N. Gu. ''Nanoparticle-reinforced resin-based dental composites''. Journal of Dentistry. Vol. 36. 2008. pp. 450-455. [ Links ]
8. S. Mitra, D. Wu, B. Holmes. ''An application of nanotechnology in advanced dental materials''. The Journal of the American Dental Association. Vol. 134. 2003. pp. 1382-1390. [ Links ]
9. J. Fu, W. Liu, Z. Hao, X. Wu, J. Yin, A. Panjiyar, et al. ''Characterization of a Low Shrinkage Dental Composite Containing Bismethylene Spiroorthocarbonate Expanding Monomer''. Int. J. Mol. Sci. Vol. 15. 2014. pp. 2400-2412. [ Links ]
10. S. Pereira, R. Osorio, M. Toledano, M. Cabrerizo, T. Nunes, S. Kalachandra. ''Novel light-cured resins and composites with improved physicochemical properties''. Dent. Mat. Vol. 23. 2007. pp. 1189-1198. [ Links ]
11. J. Antonucci, S. Dickens, B. Fowler, H. Xu, W. McDonough. ''Chemistry of Silanes: Interfaces in Dental Polymers and Composites''. J. Res. Natl. Inst. Stand. Technol. Vol. 110. 2005. pp. 541-558. [ Links ]
12. J. Matilina, L. Lassila, M. Özcan, A. Yli, P. Vallita. ''An introduction to silanes and their clinical applications in Dentistry''. Int. J. Prosthodont. Vol. 17. 2004. pp. 155-164. [ Links ]
13. T. Chen, G. Braner. ''Solvent effects on bonding organo-silane to silica surfaces''. J. Dent. Res. Vol. 61. 1982. pp. 1439-1443. [ Links ]
14. I. Sideridou, M. Karabela. ''Effect of the amount of 3-methacryloxypropyltrimethoxysilane coupling agent on physical properties of dental resin nanocomposites''. Dent. Mat. Vol. 25. 2009. pp. 1315-1324. [ Links ]
15. M. Karabela, I. Sideridou. ''Effect of the structure of silane coupling agent on sorption characteristics of solvents by dental resin-nanocomposites''. Dent. Mat. Vol. 24. 2008. pp. 1631-1639. [ Links ]
16. T. Nihei, A. Dabanoglu, T. Teranaka, S. Kurata, K. Ohashi, Y. Kondo, et al. ''Three-body-wear resistance of the experimental composite containing filler treated with hydrophobic silane coupling agents''. Dent. Mat. Vol. 24. 2008. pp. 760-764. [ Links ]
17. K. Wilson, J. Antonucci. ''Structure-property relationships in thermoset dymethacrylate nanocomposites''. Dent. Mat. Vol. 22. 2006. pp. 995-1001. [ Links ]
18. Q. Wan, J. Sheffield, J. McCool, G. Barau. ''Light curable dental composites designed with colloidal crystal reinforcement''. Dent. Mat. Vol. 24. 2008. pp. 1694-1701. [ Links ]
19. S. Beun, O. Bailly, A. Dubin, J. Vreven, J. Devaux, G. Leloup. ''Rheological properties of experimental Bis-GMA/TEGDMA flowable resin composites with various macrofiller/microfiller ratio''. Dent. Mat. Vol. 25. 2009. pp. 198-205. [ Links ]
20. S. Bean, T. Glorieux, J. Devaux, J. Vreven, G. Leloup. ''Characterization of nanofilled compared universal and microfilled composites''. Dent. Mat. Vol. 23. 2007. pp. 51-59. [ Links ]
21. A. Curtis, W. Palin, G. Fleming, A. Shortall, P. Marques. ''The mechanical properties of nanofilled resin-based composites: Characterizing discrete filler particles and agglomerates using micromanipulation technique''. Dent. Mat. Vol. 25. 2009. pp. 180-187. [ Links ]
22. M. Chen, C. Chen, S. Hsu, S. Sun, W. Su. ''Low shrinkage light curable nanocomposite for dental restorative material''. Dent. Mat. Vol. 22. 2006. pp. 138-145. [ Links ]
23. A. Dorigato, Y. Dzenis, A. Pegoretti. ''Filler aggregation as a reinforcement mechanism in polymer nanocomposites''. Mechanics of Materials. Vol. 61. 2013. pp. 79-90. [ Links ]
24. D. Strat, F. Dalmas, S. Randriamahefa, J. Jestin, V. Wintenys. ''Mechanical reinforcement in model elastomer nanocomposites with tuned microstructure and ineractions''. Polymer. Vol. 54. 2013. pp. 1466-1479. [ Links ]
25. C. DeArmitt. ''Functional Fillers for Plastics''. M. Kutz (editor). Applied Plastics Engineering Handbook. 1st ed. Ed. Elsevier Inc. Waltham, USA. 2011. pp. 455-468. [ Links ]
26. G. Kellum. ''Determination of water, silanol and strained siloxane on silica surfaces''. Analytical Chemistry. Vol. 39. 1967. pp. 341-345. [ Links ]
27. H. Bergna. ''Colloid Chemistry of Silica: An Overview''. H. Bergna, W. Roberts (editors). Colloidal Silica Fundamentals and Applications. 1st ed. Ed. Taylor & Francis Group. Boca Raton, USA. 2006. pp. 9-37. [ Links ]
28. L. Zhuravlev. ''The surface chemistry of amorphous silica. Zhuravlev model''. Colloids and Surfaces A: Physicochemical and Engineering Aspects. Vol. 173. 2000. pp. 1-38. [ Links ]
29. J. Lee, G. Um, I. Lee. ''Rheological properties of resin composites according to variations in monomer and filler composition''. Dent. Mat. Vol. 22. 2006. pp. 515-526. [ Links ]
30. T. Naganuma, Y. Kagawa. ''Effect of particle size on the optically transparent nano meter-order glass particle-dispersed epoxy matrix composites''. Composites Science and Technology. Vol. 62. 2002. pp. 1187-1189. [ Links ]
31. L. Zhou, H. Zhang, H. Zhang, Z. Zhang. ''Homogeneous nanoparticle dispersion prepared with impurity-free dispersant by the ball mill technique''. Particuology. Vol. 11. 2013. pp. 441-447. [ Links ]
32. X. Zhang, Y. Li, G. Lv, Y. Zuo, Y. Mu. ''Thermal and crystallization studies of nano-hidroxyapatite reinforced polyamide 66 biocomposites''. Polymer degradation and stability. Vol. 91. 2006. pp. 1202-1207. [ Links ]