Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230
Rev.fac.ing.univ. Antioquia no.76 Medellín July/Sept. 2015
https://doi.org/10.17533/udea.redin.n76a10
ARTÍCULO ORIGINAL
DOI: 10.17533/udea.redin.n76a10
Cation and anion monitoring in a wastewater treatment pilot project
Monitoreo de catión y anión en el proyecto piloto de tratamiento de aguas residuales
Magda de Almeida1, Filipe Vargas-Zerwes2, Lucas Ferreira-Bastos1, Adilson Ben da Costa3,4, Rosana de Cassia de Souza-Schneider1,2*, Ênio Leandro Machado1,2, Andreas Kohler3
1Departamento de Química e Física, Universidade de Santa Cruz do Sul. Av. Independência, 2293. CEP: 96815-900. Santa Cruz do Sul, Brasil.
2Programa de Pós-graduação em Tecnologia Ambiental, Universidade de Santa Cruz do Sul. Av. Independência, 2293, CEP: 96815-900. Santa Cruz do Sul, Brasil.
3Departamento de Biologia e Farmácia, Universidade de Santa Cruz do Sul. Av. Independência, 2293, CEP 96815-900. Santa Cruz do Sul, Brasil.
4Programa de Pós-graduação em Sistemas e Processos Industriais, Universidade de Santa Cruz do Sul. Av. Independência, 2293, CEP 96815-900. Santa Cruz do Sul, Brasil.
* Corresponding author: Rosana de Cassia de Souza Schneider, e-mail: rosana@unisc.br
DOI: 10.17533/udea.redin.n76a10
(Received September 2014; accepted April 20, 2015)
ABSTRACT
The purpose of wastewater treatment is water reuse. It reduces potable water consumption while preventing fresh water contamination. Water reuse schemes have already been successfully established in different locations. Treatments using constructed wetlands are widely studied as a more economical and environmentally-friendly alternative for treating wastewater. In these systems, the control of inorganic species is also important. This study monitored cations (Na+, K+, Li+ and NH4+) and anions (SO42-, NO3-, NO2-, Cl- and PO43-) in a constructed wetlands (CWs) system, a rainwater catchment system, sewage treatment system, and in final reuse water. The monitoring was accomplished using ion chromatographic analysis. The removal values found in the CWs were: 99.9% K+, NH4+ and SO42-, 52.6% Na+, 89.8% NO3-, 98.2% NO2-, 63.6% Cl- and 96.8% PO43-. The results also showed that CWs system is suitable for removing ions from the wastewater.
Keywords: Reuse, constructed wetlands (CWs), wastewater monitoring, ions
RESUMEN
El propósito del tratamiento de aguas residuales es la reutilización del agua. Esta reduce el consumo de agua potable y previene la contaminación del agua de primer uso. La reutilización del agua ya se ha implementado con éxito en diferentes lugares. Los tratamientos que utilizan los humedales artificiales son ampliamente estudiados como una alternativa más económica y ecológica para tratar las aguas residuales. En estos sistemas, el control de especies inorgánicas también es importante. Este estudio ha monitoreado cationes (Na+, K+, Li+ y NH4+) y aniones (SO42-, NO3-, NO2-, Cl- y PO42-) en un sistema de humedales construido (CWs), en un sistema de captación de agua de lluvia, en el tratamiento de aguas residuales y en agua reutilizable final. El monitoreo se llevó a cabo utilizando el análisis cromatográfico de iones. Los valores de remoción encontrados en CWs fueron: 99,9% K+, NH4+ y SO42-, 52,6% Na+, 89,8% NO3-, 98,2% NO2-, 63,6% Cl- y 96,8% PO42-. Los resultados también mostraron que el sistema CWs está adecuado para la eliminación de iones del agua residual.
Palabras clave: Reutilización, humedales artificiales, monitoreo de aguas residuales, iones
1. Introduction
Environmental problems and restrictions indicate the necessity of planned water reuse. For more than 20 years, scientists have been seeking ways to avoid the shortage of water resources and control the rising cost of drinking water [1-9].
The estimated number of people living in regions with predicted intense water scarcity in 2025 is approximately 2.7 billion people, and the volume of water will have to increase 41% to meet the needs of the population [7]. In poor countries, the problems are more alarming in relation to untreated wastewater [10].
Even though most of the farm households are aware of the environmental and health consequences related to the crop irrigation with wastewater, they still use it because of its cost advantages. This issue is one of the sanitary challenges also found at water-scarce regions [11]. This practice is widely used in Africa; for example, in [12] observed that wastewater treatment systems do not always reach enough quality for the purposes of irrigation and for non-potable applications. Therefore, water reuse might be dangerous for the farmers in some regions.
An alternative for minimizing the water scarcity is the reuse of sewage water from washing clothes and dishes as well as from showers because it is non-potable water. This procedure has already been successfully introduced in hotels [3], schools [8] and residential buildings [13].
The cost reduction of the water reuse process has been studied to find a more economic and natural method. Constructed wetlands (CWs) are an alternative eco-technological system for small-scale communities [14].
A wastewater treatment system using CWs has minimum operating costs and may solve the problems concerning wastewater in rural areas with low incomes [15]. According to [16], CWs have been the simplest and least costly alternative for wastewater treatment in rural areas in China. These CWs are promising systems for the removal of carbon and nitrogen. However, the nitrogen removal may not be as effective due to low dissolved oxygen in wetlands. Using CWs may also be an alternative in rural domestic wastewater for phosphorus removal [17] as well as to reduce 99.9 % of fecal coliforms [18].
Thus, the CWs have several advantages such as high efficiency purification, robust system, plasticity of configuration with different plant species, ability to adapt to load changes, low cost of engineering infrastructure and operation, etc. [18, 19]. Moreover, they can be integrated into productive agricultural systems. For example, better yields are reached planting rice with this system when comparing to the use of fertilizers [17].
In studies conducted in cold regions of China, the type of crop used can ensure the development of CWs systems even in cold periods [20] because the thick layer of biomass may provide thermal insulation. Otherwise, the system would be more complex requiring subsurface drainage with a greenhouse structure [21]. In [20] used a CWs system with Salix babylonica and they obtained the thermal insulation. Moreover, they concluded that it is profitable, does not require energy input and it may be used in single-family homes in developing regions.
Furthermore, the coupling of an upflow anaerobic sludge blanket (UASB) prior to CWs is a promising alternative to traditional septic/sink tank systems that are widely used for domestic wastewater [22].
The vegetation choice is essential in the CWs and has the function of absorbing pollutants and transferring nutrients from microorganisms in the rhizosphere [23]. In this system, there is a combination of continuous exposure to flooding and waste streams containing a relatively high concentration and variety of pollutants [24].
Several types of vegetation have already been studied, and their absorption efficiency for many different nutrients is known [23, 25-28].
The absorbed nutrient rate by plants varies from 3 to 47% for nitrogen removal and 3 to 60% for phosphorus removal. This variation is determined by the type of plant used in the treatment and the type of effluent to be treated [26].
Several studies have demonstrated the efficacy of CW treatment with respect to fecal coliform and pathogenic micro-organism removal [3, 4]. In addition, the biochemical oxygen demand (BOD) and chemical oxygen demand (COD) removal were approximately 80% [29]. The excessive concentration of nutrients in wastewater, particularly nitrogen and phosphorus leads to the eutrophication of water bodies (such as, lakes and rivers) and subterranean water contamination [30-32].
CW treatments also show good efficiency in removing micronutrients, which is highlighted by the removal of aluminum (90%) and zinc (78%) [33].
The high presence of some ions may harm plants, animals and humans, the ammonium ion in high concentration may cause visible foliar injury. Furthermore, when the assimilation of this ion by plants is high, a significant increase of nitrogen in organic tissue may occur. Excessive absorption of ammonia and ammonium ions by the plant in addition to changing the biomass growth also modifies the sensitivity to drought and frost resistance [34]. Additionally, high concentrations of ammonium ion in water bodies can be toxic to aquatic organisms and ultimately to humans when converted to nitrate [35].
Contamination with high concentrations of chloride ions in water can damage metal pipes and concrete structures [36].
In domestic wastewater, it is common to find high concentrations of chloride and sodium because of the human diet and the composition of cleaning products such as soap. The chloride tends to remain constant through traditional processing of effluents; however, it is known that it may be decreased by ion exchange and reverse osmosis. Water containing residual chlorine with concentrations greater than 10 mg L-1 can harm agricultural crops [30] in the same way as the reuse of water with high concentrations of sodium may cause handling problems in crops [37]. Additionally, studies show that sodium has harmful effects on soil, resulting in toxic effects for the plants [38, 39].
The alarming toxicity caused by lithium is equivalent to uranium and selenium in the early life stages of some fishes [40]. Lithium in wastewater is linked to the chemical composition of antidepressant drugs ingested by the population [41]. This type of medicine has been increasingly used in the Rio Pardo Valley region due to chronic exposure to pesticides that can increase cases of neurobehavioral disorders such as anxiety and depression [42].
On the other hand, it is possible to incorporate water from the reuse system with rainwater for use in residences and buildings. Rainwater is less polluted, and it is usually available in quantities sufficient for the processes of filtration, sedimentation and disinfection for an efficient treatment [5]. Rainwater is generally used for non-potable purposes such as for laundry, washing cars or gardening, though treated rainwater can be used for more noble purposes, even for drinking [9]. Incorporating the collected rainwater into reusable water from sludge allows for the optimization of the system and production of sufficient water quantities for building use [5].
Therefore, this study monitored cations (Na+, K+, Li+ e NH4+) and anions (SO42-, NO3-, NO2-, Cl- e PO42-) in a constructed wetland system (CWs) for a catchment system, in sewage treatment, and in the final reusable water, as well as evaluated rainwater as a water resource in the system. The studied treatment will contribute to water reuse for small farmers in the Brazilian countryside, mainly for non-potable applications such as toilet flushing with greywater recycled. It is highlighted that monitoring ions is a helpful tool to determine the efficiency of wastewater treatment for small farms. In this way, it is also assessed if these ions are increasing after water treatment and in the reuse water.
2. Material and Methods
2.1. Rainwater capture and wastewater treatment pilot project
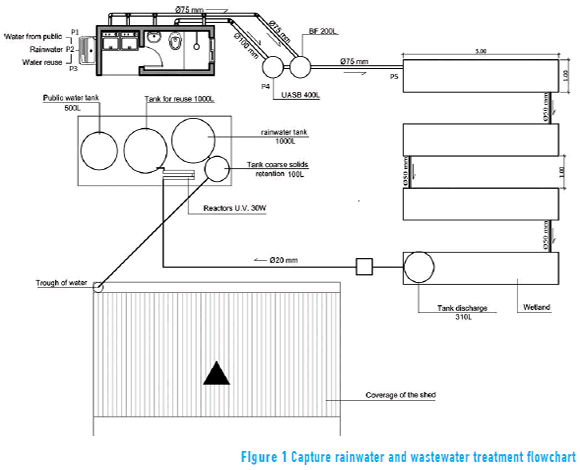
The project was established on a small farm in the Vera Cruz town, Rio Grande do Sul, Brazil. The aim is the treatment of two categories of water: rainwater, wastewater (from washing clothes, showers, the bathroom sink and flushing the toilet). Each type of water receives a special treatment. The sample collection points and treatment are detailed in Figure 1.
Collection of rainwater was through the house's roof gutters. For the retention of solids, this water was driven to a 100 L tank for retention of solids, which was connected to another tank of 1000 L that was intended for the temporary storage of rainwater. Water from the temporary reservoir was disinfected, a process in which it went through two ultraviolet lamps (30 W). Rainwater after treatment was used for flushing the toilet, washing clothes and garden watering.
The wastewater from washing clothes, the shower and the bathroom sink were directed to an anaerobic filter through an independent pipeline of wastewater from toilet.
Blackwater generated exclusively from flushing the toilet was treated in the UASB reactor and subsequently in the anaerobic filter. In the anaerobic filter, gray and black water were homogenized, both being designed to remove nutrients and pathogens in a sequenced CW.
The CWs were 20 m² and were divided into four sequential tanks with 5 m² (1 m x 5 m) dimensions. The vegetation used for phytoremediation was identified as an aquatic macrophyte Hymenachne grumosa.
The water was stored in a 310 L tank and then pumped into a reactor for disinfection by ultraviolet radiation. After treatment the effluent was stored in a 1000 L tank for further use for flushing the toilet.
2.2. Ion monitoring
The samples were obtained at six different sites: municipal water (from another point in the residence), collected rainwater, into the UASB, first wetland input, last wetland output and reusable water (final of treatment). The samples were stored at 8 °C, filtered with syringe filters (0.45 µm) and analyzed for 24 h. The chromatographic analyses were performed in triplicate.
The ion chromatography equipment (Model 883, Metrohn) was used with a conductivity detector. For the determination of anions, a Metrosep A Supp 5 column (150 mm x 4 mm x 5 µm) was used with a 3.2 mmol L-1 sodium carbonate and 1.0 mmol L-1 sodium bicarbonate solution as the mobile phase.
For the cation analysis, a Metrosep C 4 (150 mm x 4 mm x 5 µm) column was used with 1.7 mmol L-1 nitric acid and 0.7 mmol L-1 dipicolinic acid solutions as the mobile phase.
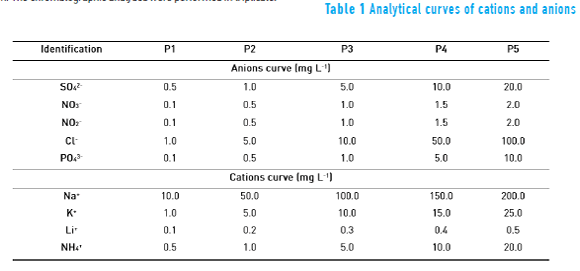
Ion quantification was performed by external standardization from the reference solutions with 1000 mg L-1 for each ion in ultrapure water. Two curves were prepared, one for the cations (Na+, K+, Li+ and NH4+) and the other one for the anions (SO42-, NO3-, NO2-, Cl- and PO43-) as shown in Table 1. Detection (LOD) and quantification (LOQ) limits were determined [43].
Samples collected at the UASB reactor could not be analyzed by ion chromatography because of high levels of organic matter. These samples were analyzed according to the methods described in Standard Methods for the Examination of Water and Wastewater [44].
Sodium and potassium ions were determined by flame photometry (B462, Micronal), sulfate ions were determined by turbidimetry (600 plus, Femto), nitrite and nitrate ions were determined by spectrophotometry in the visible region (Specord PLUS 2010, Analytik Jena) and chloride ions were determined by titrimetry.
2.3. Evapotranspiration
Evapotranspiration data were obtained from the Automatic Weather Station (Davis brand and Vantage Pró Plus model) at Santa Cruz do Sul University, which records the data averages every 30 min via the Weather Link 5.9 software and it is located less than 8 miles from the property where the experiment was installed.
3. Results and discussions
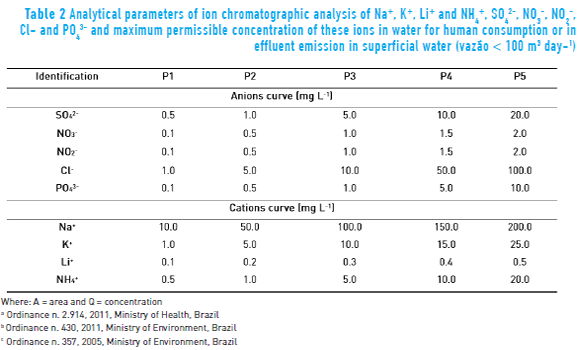
The ion chromatographic results were reliable with a good determination coefficient (R2) and quantification limits (LOQ). These results were less than the values established in the 2914 ordinance of the Ministry of Health [45] which determines the allowable limits for human consumption and also less than the 357 and 430 ordinances of Ministry of Environment for discharge of final effluent. Even if water after the CWs can be reused, there is no specific ordinances stating allowable limits. That is why the comparison was made with the cited ordinances. Table 2 details the limits, values for LOD and LOQ as well as the equations of the straight line and the correlation coefficients for each ion by ion chromatography. Although the limit for sodium content is for drinking water purposes and there are no limits displayed for potassium, the monitoring of these ions helps to judge the efficiency of CWs.
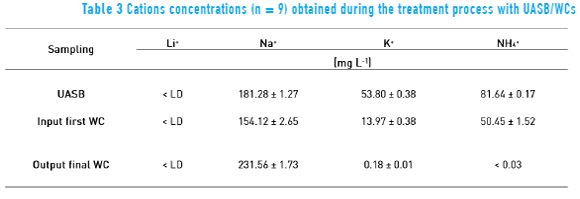
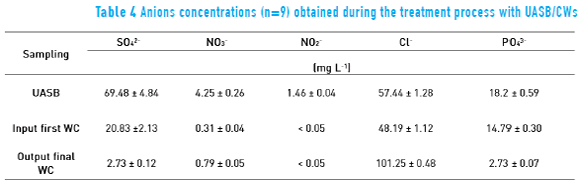
Tables 3 and 4 list the average results for each treatment stage after CWs is fully developed.
Water reuse results of the system evaluation period showed that the CW development was important for the effluent ion removal. Efficiency of removing potassium and ammonia reached a maximum of 99.9% for both cations, with an average efficiency of 98.4% and 99.3%, respectively.
Sodium ions increased after the CWs. This behavior was also observed for the UASB/CWs system applying Hymenachne grumosa from 63.0 to 81.9 mg L-1 of sodium ion. The increased sodium concentration may be linked to a lower efficiency of the root system ion exchange and, more markedly, evapotranspiration. If the concentration of sodium increases, it can be removed using other process, as applied with industrial wastewater [46] or diluted with rain water according to the observations made in the experiments. In rainy seasons, this problem was minimized.
Potassium removal was effective in CWs, as shown in Table 3. The potassium ion is a macronutrient essential for plant growth [47].
The results were consistent with those obtained by removing ammonia in graywater employing zeolite (aluminosilicate tetrahedral) with negative charge on the framework [48]. Moreover, if the system is used with plants of high nutrient absorption, the removal of ammonia, phosphate and nitrates could be major [49].
Such as the cations, anions were also removed by CW treatment (Table 4). Comparing the results from initial effluent and final reusable water, phosphate was reduced up to 96.3%. The main mechanisms of phosphorus removal by CWs system are adsorption, complexation, precipitation, absorption and assimilation by the plant [50].
CW phytoremediation is indicated for the control of phosphorus discharged from municipal and industrial wastewater treatment plants, which is a key factor in preventing eutrophication of surface waters. Usually, the incorporation in biological systems prevents the removal of phosphorous by the addition of flocculants and also by the operation of solid separation [51]. The results found for phosphorus removal showed how Hymenachne grumosa is effective for phosphorus removal as it was observed by [52].
Nitrate, nitrite and sulfate reduction were 63.1, 95.1 and 96.9%, respectively. Furthermore, chloride ions, such as sodium ions, increased in the treatment. These ions are from household cleaning products. We highlighted that the nitrates did not decrease more because the ammonium ion may have been fixed as nitrate [24, 50].
The condition of anoxic region was probably not favoured in the output final WC due to the lower concentration of nitrate ions which reduces the action of denitrifying microorganisms [53].
Some ions in the wastewater do not interact with roots of the wetland as nitrogen and phosphorous compounds do. Chloride, sodium and potassium display elevated values in the discharge of the wastewaters. Chloride, especially, moves freely through the wetland to the outlet streams. This is also confirmed by the studies of [54], where the recovery of sodium ions from the tributaries of small rural communities were between 70-82% in the effluents.
In addition, other plants can be tested for sodium and choride removal. As [55] halophyte plants are able to reduce the salinity of wastewater by accumulating salts in their tissues.
4. Conclusions
Ion chromatography was an excellent tool for ion monitoring in wastewater. With the CWs using Hymenachne grumosa, the phytoremediation was powerful, reaching removal of 99.9% K+, NH4+ and SO42-; 98.2% NO3-, 89.8% NO2- and 96.8% PO43-. Cl- and Na+ were in high concentration in the reuse system. The proposed CWs are not advisable for Na+ and Cl- removal. In relation to ion concentration, the reuse system with UASB, CWs and rainwater input was efficient and suitable for small farms.
5. Acknowledgements
We would like to thank SCIT – RS, FAPERGS (11/1476-9), FAP-UNISC and CNPq (306178/2012-5). We gratefully acknowledge the farmers that participated in this study.
6. References
1. D. Baumann. "Social acceptance of water reuse". Applied Geography. Vol. 3. 1983. pp. 79-84. [ Links ]
2. O. Al-Jayyousi. "Greywater reuse: towards sustainable water management". Desalination. Vol. 156. 2003. pp. 181-192. [ Links ]
3. J. March, M. Gual, F. Orozco. "Experiences on greywater re-use for toilet flushing in a hotel (Mallorca Island, Spain)". Desalination. Vol. 164. 2004. pp. 241-247. [ Links ]
4. M. Greenway. "The role of constructed wetlands in secondary effluent treatment and water reuse in subtropical and arid Australia". Ecological Engineering. Vol. 25. 2005. pp. 501-509. [ Links ]
5. R. Kim, S. Lee, J. Jeong, J. Lee, Y. Kim. "Reuse of greywater and rainwater using fiber filter media and metal membrane". Desalination. Vol. 202. 2007. pp. 326-332. [ Links ]
6. M. Ortiz, R. Raluy, L. Serra. "Life cycle assessment of water treatment technologies: wastewater and water-reuse in a small town". Desalination. Vol. 204. 2007. pp. 121-131. [ Links ]
7. A. Urkiaga, L. Fuentes, B. Bis, E. Chiru, B. Balasz. F. Hernández. "Development of analysis tools for social, economic and ecological effects of water reuse". Desalination. Vol. 218. 2008. pp. 81-91.
8. S. Godfrey, P. Labhasetwar, S. Wate. "Greywater reuse in residential schools in Madhya Pradesh, India-A case study of cost–benefit analysis". Resources, Conservation and Recycling. Vol. 53. 2009. pp. 287-293. [ Links ]
9. Z. Li, F. Boyle, A. Reynolds. "Rainwater harvesting and greywater treatment systems for domestic application in Ireland". Desalination. Vol. 260. 2010. pp. 1-8. [ Links ]
10. A. Kivaisi. "The potential for constructed wetlands for wastewater treatment and reuse in developing countries: a review". Ecological Engineering. Vol. 16. 2001. pp. 545-560. [ Links ]
11. M. Qadir, D. Wichelns, L. Raschid, P. McCornick, P. Drechsel, A. Bahri, P. Minhas. "The challenges of wastewater irrigation in developing countries". Agricultural Water Management. Vol. 97. 2010. pp. 561-568. [ Links ]
12. J. Kihila, K. Mtei, K. Njau. "Wastewater treatment for reuse in urban agriculture; the case of Moshi Municipality, Tanzania". Physics and Chemistry of the Earth, Parts A/B/C. Vol. 72-75. 2014. pp. 104-110. [ Links ]
13. M. Goddard. "Urban greywater reuse at the D'LUX Development". Desalination. Vol. 188. 2006. pp. 135-140. [ Links ]
14. M. Yildirim, B. Topkaya. "Assessing Environmental Impacts of Wastewater Treatment Alternatives for Small-Scale Communities". CLEAN – Soil, Air, Water. Vol. 40. 2012. pp. 171-178. [ Links ]
15. K. Gunes. "Restaurant Wastewater Treatment by Constructed Wetlands". CLEAN – Soil, Air, Water. Vol. 35. 2007. pp. 571-575. [ Links ]
16. F. Ye, Y. Li. "Enhancement of nitrogen removal in towery hybrid constructed wetland to treat domestic wastewater for small rural communities". Ecological Engineering. Vol. 35. 2009. pp. 1043-1050. [ Links ]
17. S. Li, H. Li, X. Liang, Y. Chen, S. Wang, F. Wang. "Phosphorus removal of rural wastewater by the paddy-rice-wetland system in Tai Lake Basin". Journal of Hazardous Materials. Vol. 171. 2009. pp. 301-308. [ Links ]
18. L. Xu, H. You, J. Li, Q. Zhang, J. Jiang, L. Weiming, Z. Sun. "Analysis on Affected Factors of Treatment Efficiency of Rural Sewage Removal with Constructed Wetland". Procedia Environmental Sciences. Vol. 10. 2011. pp. 2314-2319. [ Links ]
19. P. Kuschk, A. Wießner, U. Kappelmeyer, E. Weißbrodt, M. Kästner, U. Stottmeister. "Annual cycle of nitrogen removal by a pilot-scale subsurface horizontal flow in a constructed wetland under moderate climate". Water Research. Vol. 37. 2003. pp. 4236-4242. [ Links ]
20. S. Wu, D. Austin, L. Liu, R. Dong. "Performance of integrated household constructed wetland for domestic wastewater treatment in rural areas". Ecological Engineering. Vol. 37. 2011. pp. 948-954. [ Links ]
21. D. Gao, Q. Hu. "Bio-contact oxidation and greenhouse-structured wetland system for rural sewage recycling in cold regions: A full-scale study". Ecological Engineering. Vol. 49. 2012. pp. 249-253. [ Links ]
22. S. Mbuligwe. "Comparative effectiveness of engineered wetland systems in the treatment of anaerobically pre-treated domestic wastewater". Ecological Engineering. Vol. 23. 2004. pp. 269-284. [ Links ]
23. L. Wang, H. Gan, F. Wang, X. Sun, Q. Zhu. "Characteristic Analysis of Plants for the Removal of Nutrients from a Constructed Wetland using Reclaimed Water". CLEAN – Soil, Air, Water. Vol. 38. 2010. pp. 35-43. [ Links ]
24. B. Zhang, J. Zheng, R. Sharp. "Phytoremediation in Engineered Wetlands: Mechanisms and Applications". Procedia Environmental Sciences. Vol. 2. 2010. pp. 1315-1325. [ Links ]
25. L. Yang, H. Chang, M. Huang. "Nutrient removal in gravel- and soil-based wetland microcosms with and without vegetation". Ecological Engineering. Vol. 18. 2001. pp. 91-105. [ Links ]
26. N. Gottschall, C. Boutin, A. Crolla, C. Kinsley, P. Champagne. "The role of plants in the removal of nutrients at a constructed wetland treating agricultural (dairy) wastewater, Ontario, Canada". Ecological Engineering. Vol. 29. 2007. pp. 154-163. [ Links ]
27. J. Iamchaturapatr, S. Yi, J. Rhee. "Nutrient removals by 21 aquatic plants for vertical free surface-flow (VFS) constructed wetland". Ecological Engineering. Vol. 29. 2007. pp. 287-293. [ Links ]
28. S. Liu, B. Yan, L. Wang. "The layer effect in nutrient removal by two indigenous plant species in horizontal flow constructed wetlands". Ecological Engineering. Vol. 37. 2011. pp. 2101-2104. [ Links ]
29. I. Kotti, G. Gikas, V. Tsihrintzis. "Effect of operational and design parameters on removal efficiency of pilot-scale FWS constructed wetlands and comparison with HSF systems". Ecological Engineering. Vol. 36. 2010. pp. 862-875. [ Links ]
30. V. Emongor, G. Ramolemana. "Treated sewage effluent (water) potential to be used for horticultural production in Botswana". Physics and Chemistry of the Earth, Parts A/B/C. Vol. 29. 2004. pp. 1101-1108. [ Links ]
31. P. Nyenje, J. Foppen, S. Uhlenbrook, R. Kulabako, A. Muwanga. "Eutrophication and nutrient release in urban areas of sub-Saharan Africa - A review". Science of The Total Environment. Vol. 408. 2010. pp. 447-455. [ Links ]
32. H. Wu, J. Zhang, P. Li, J. Zhang, H. Xie, B. Zhang. "Nutrient removal in constructed microcosm wetlands for treating polluted river water in northern China". Ecological Engineering. Vol. 37. 2011. pp. 560-568. [ Links ]
33. L. Kröpfelová, J. Vymazal, J. Svehla, J. Stíchová. "Removal of trace elements in three horizontal sub-surface flow constructed wetlands in the Czech Republic". Environmental Pollution. Vol. 157. 2009. pp. 1186-1194. [ Links ]
34. S. Krupa. "Effects of atmospheric ammonia (NH3) on terrestrial vegetation: a review". Environmental Pollution. Vol. 124. 2003. pp. 179-221. [ Links ]
35. X. Dong, G. Reddy. "Ammonia-oxidizing bacterial community and nitrification rates in constructed wetlands treating swine wastewater". Ecological Engineering. Vol. 40. 2012. pp. 189-197. [ Links ]
36. P. Rolf. Reações de química na análise de água. 1st ed. Ed. Arte Visual. Fortaleza, Brasil. 2009. pp. 1-334. [ Links ]
37. G. Hussain, A. Al-Saati. "Wastewater quality and its reuse in agriculture in Saudi Arabia". Desalination. Vol. 123. 1999. pp. 241-251. [ Links ]
38. R. Rodrigues, V. Júnior, J. Lollo. "Influência dos constituintes do esgoto no colapso de um solo arenoso". Eng. Sanit. Ambient. Vol. 15. 2010. pp. 29-36. [ Links ]
39. C. Rodrigues, J. Silveira, E. Silva, A. Dutra, R. Viégas. "Transporte e distribuição de potássio atenuam os efeitos tóxicos do sódio em plantas jovens de pinhão-manso". Rev. Brasileira de Ciência do Solo. Vol. 36. 2012. pp. 223-232. [ Links ]
40. V. Tkatcheva, I. Holopainen, H. Hyvärinen, J. Kukkonen. "The responses of rainbow trout gills to high lithium and potassium concentrations in water". Ecotoxicology and Environmental Safety. Vol. 68. 2007. pp. 419-425. [ Links ]
41. F. Souza. "Tratamento da depressão". Revista Brasileira de Psiquiatria. Vol. 21. 1999. pp. 18-23. [ Links ]
42. V. Etges, M. Ferreira. A produção de tabaco: impacto no ecossistema e na saúde humana na região de Santa Cruz do Sul/RS. 1st ed. Ed. EDUNISC. Santa Cruz do Sul, Brasil. 2006. pp. 1-248. [ Links ]
43. D. Skoog, F. Holler, T. Nieman. Principles of Instrumental Analysis. 5th ed. Ed. Saunders College Pub. California, USA. 1998. pp. 1-849. [ Links ]
44. American Public Health Association, Water Environment Federation. Standard Methods for the Examination of Water & Wastewater. 21st ed. Ed. American Public Health Association. Washington, USA. 2005. pp. 1-55. [ Links ]
45. Ministério da Saúde. Portaria 2914: Dispõe sobre os procedimentos de controle e de vigilância da qualidade da água para consumo humano e seu padrão de potabilidade. Diário Oficial da União, Seção 1 de 14 de dezembro de 2011. Brasilia, Brasil. 2011. pp. 1-7. [ Links ]
46. N. Melián, J. Sadhwani, S. Pérez. "Saline waste disposal reuse for desalination plants for the chlor-alkali industry: The particular case of pozo izquierdo SWRO desalination plant". Desalination. Vol. 281. 2011. pp. 35-41. [ Links ]
47. E. Viana, J. Kiehl. "Doses de nitrogênio e potássio no crescimento do trigo". Bragantia. Vol. 69. 2010. pp. 975-982. [ Links ]
48. N. Widiastuti, H. Wu, H. Ang, D. Zhang. "Removal of ammonium from greywater using natural zeolite". Desalination. Vol. 277. 2011. pp. 15-23. [ Links ]
49. F. Yao, J. Sun, C. Tang, W. Ni. "Kinetics of Ammonium, Nitrate and Phosphate Uptake by Candidate Plants Used in Constructed Wetlands". Procedia Environmental Sciences. Vol. 10. Part B. 2011. pp. 1854-1861. [ Links ]
50. X. Zhang, P. Liu, Y. Yang, W. Chen. "Phytoremediation of urban wastewater by model wetlands with ornamental hydrophytes". Journal of Environmental Sciences. Vol. 19. 2007. pp. 902-909. [ Links ]
51. S. Ojoawo, G. Udayakumar, P. Naik. "Phytoremediation of Phosphorus and Nitrogen with Canna x generalis Reeds in Domestic Wastewater through NMAMIT Constructed Wetland". Aquatic Procedia. Vol. 4. 2015. pp. 349-356. [ Links ]
52. T. Horn, F. Zerwes, L. Kist, Ê. Machado. "Constructed wetland and photocatalytic ozonation for university sewage treatment". Ecological Engineering. Vol. 63. 2014. pp. 134-141. [ Links ]
53. C. Lee, T. Fletcher, G. Sun. "Nitrogen removal in constructed wetland systems". Engineering in Life Sciences. Vol. 9. 2009. pp. 11-22. [ Links ]
54. G. Merlin, J. Pajean, T. Lissolo. "Performances of constructed wetlands for municipal wastewater treatment in rural mountainous area". Hydrobiologia. Vol. 469. 2002. pp. 87-98. [ Links ]
55. O. Shelef, A. Gross, S. Rachmilevitch. "The use of Bassia indica for salt phytoremediation in constructed wetlands". Water Research. Vol. 46. 2012. pp. 3967-3976. [ Links ]