Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230
Rev.fac.ing.univ. Antioquia no.76 Medellín July/Sept. 2015
https://doi.org/10.17533/udea.redin.n76a11
ARTÍCULO ORIGINAL
DOI: 10.17533/udea.redin.n76a11
Sinú River raw water treatment by natural coagulants
Tratamiento de agua cruda del Río Sinú con extractos coagulantes naturales
Johana Paola Rodiño-Arguello1, Jhon Jairo Feria-Diaz3*, Roberth de Jesús Paternina-Uribe2, José Luis Marrugo-Negrete2
1Grupo de investigación en Calidad de Aguas y Modelación Hídrica y Ambiental (CAMHA), Universidad Pontificia Bolivariana. Carrera 6 n.o 97A-99. C. P. 230002. Montería, Colombia.
2Grupo de investigación en Aguas, Química Aplicada y Ambiental (AQAA), Universidad de Córdoba. Carrera 6 n.o 76-103. C. P. 230002. Montería, Colombia.
3Grupo de investigación en Medio Ambiente y Aguas (GIMAGUAS), Universidad de Sucre. Carrera 28 n.o 5-267. C.P.700001. Sincelejo, Colombia.
* Corresponding author: Jhon Jairo Feria Diaz, e-mail: jhon.feria@gmail.com
DOI: 10.17533/udea.redin.n76a11
(Received November 28, 2014; accepted June 04, 2015)
ABSTRACT
Five natural coagulants extracts in saline were evaluated: stems Hylocereus cf. trigonus (Cactus), exudate gum Albizia saman (Campano), bark Guazuma ulmifolia (Guácimo) and bark and seed of Moringa oleífera (Moringa) in raw water samples taken from the Sinú river with initial turbidity levels between 56 and 300 nephelometric turbidity units (NTU). With Jar tests, the turbidity removal efficiencies as a function of percent activity coagulant dosages applied between 5 mg/L to 200 mg/L was determined. Although the Total Organic Carbon (TOC) is an important parameter of the water quality, it was not included in this study because it has been found that the Sinú river turbidity is from sedimentary origin and its stream has a low organic load. The optimal extract dosage was found to be between 10 mg/L and 40 mg/L obtaining removal efficiencies from 40% (turbidity lower than 100 NTU) to 90% (initial turbidity higher than 150 NTU) for extracts of stems H. cf. trigonus, exudate gum A. saman, bark G. ulmifolia and bark M. oleífera. The M. oleífera seed extract had the greatest turbidity removal efficiency even when using an initial turbidity higher than 150 NTU, achieving a coagulant activity up to 98%.
Keywords: Sinú river, Hylocereus cf. trigonus, Albizia saman, Guazuma ulmifolia, Moringa oleífera, coagulant activity
RESUMEN
Se evaluaron cinco extractos coagulantes naturales en solución salina de tallos de Hylocereus cf. trigonus (Cactus), exudado gomoso de Albizia saman (Campano), corteza de Guazuma ulmifolia (Guácimo) y corteza y semilla de Moringa oleífera (Moringa), en muestras de agua cruda tomadas del río Sinú, con niveles de turbidez inicial entre 56 y 300 unidades nefelométricas de turbidez (UNT). Con ensayos de jarras, se determinó las eficiencias de remoción de turbidez, como una función del porcentaje de actividad coagulante, para dosis aplicadas entre 5 mg/L a 200 mg/L. Aunque el Carbono Orgánico Total (COT) es un parámetro importante en la calidad del agua, no se incluyó en este estudio debido a que se ha hallado que la turbidez del rio Sinú es de origen sedimentario y su corriente tiene una baja carga orgánica. Las eficiencias de remoción variaron de 40% (con turbidez menores a 100 UNT) hasta 90% (con turbidez inicial mayor a 150 UNT), para dosis óptimas de 10 mg/L a 40 mg/L de extractos de tallos de H. cf. trigonus, exudado gomoso de A. saman, y cortezas de G. ulmifolia y de M. oleífera. El extracto de mayor eficiencia fue el obtenido de la semilla de M. oleífera, siendo más efectivo con turbidez mayor a 150 UNT, logrando una actividad coagulante hasta del 98%.
Palabras clave: Río Sinú, Hylocereus cf. trigonus, Albizia saman, Guazuma ulmifolia, Moringa oleífera, actividad coagulante
1. Introduction
Access to drinking water and safety sanitation systems for many communities around the world is limited. According to the United Nation Children's Fund –UNICEF, around 15% of the world population does not have drinking water [1-3], in addition, the World Health Organization –WHO established that at least 11% of world population, equivalent to 783 million of people, does not have access to drinking water and thousands of millions do not receive sanitation services in spite of the accomplishment of the millennium development goal of halving the proportion of people without access to drinking water before the year 2015 [4]. In Colombia just 11.8% of the rural sector has drinking water supply [5], while the urban sector has 97, 6% of aqueduct coverage [6].
The aluminum sulfate and ferric chloride have been traditionally used as primary coagulants in clarification and potabilization processes of the raw water [7] even though municipalities from the Colombian Atlantic coast do not have a drinking water supply with adequate treatment [8]. The usage of the current coagulants has disadvantages associated with the high acquisition costs, the production of high volumes of sludge, changes of water pH and alkalinity [9, 10], possible relation with Alzheimer and some kinds of cancer [11, 12], problems that could be minimized using natural coagulants that can be extracted from plants and animals, as well as some microorganisms [1, 7, 13, 14].
Natural coagulants are mostly carbohydrates (polysaccharides) and proteins [15]. They are polymeric compounds that can have even ionic or no ionic character (cations or anions), where the ionic ones are commonly known as polyelectrolytes. The principal advantages of the implementation of natural coagulants are the following: Organic and inorganic turbidity removal, reduction of true and apparent color, production of easy to deal with sludge, destruction of pathogens, algae and planktons, as well as the elimination of substances imparting odor and flavor. Natural coagulants usage is profitable since the treatment low costs, the steady pH levels in the treated water and because they are highly biodegradable. These advantages are even better if the plants used to extract the coagulant are autochthonous from the rural communities [16, 17]. Generally, the mechanisms followed by natural coagulants are ruled by the absorption processes and the subsequent charge neutralization or polymeric bridge effect [15].
Saline extracts of natural coagulants (SEC) of the following: stem of Hylocereus cf. trigonus (Cactus), exudate gum of Albizia saman (campano), bark of Guazuma ulmifola (Guácimo) and bark and seed of Moringa oleífera (Moringa) were used in order to evaluate their efficiency for removing raw water turbidity of the Sinú river; which is important since this river is the principal source providing water to the aqueduct systems in the department of Córdoba (Colombia); however, its turbidity levels are higher than 1,200 NTU, during the rainy season, and even higher than 40 NTU, during the dry season [18].
2. Experimental
2.1. Sampling of raw water
Raw water samples for this study were collected from the Sinú river at the neighborhood of Mocarí, municipality of Monteria, Department of Córdoba, Colombia; between the months of November of 2013 and June of 2014, for a total of five simple samplings that included both dry and rainy seasons.
2.2. Collection and selection of plant material
Plant material was selected considering the information of previous studies where they have shown good properties such as: coagulant activity, availability and nutrient composition, especially the content of protein and carbohydrates [17], as the ones that had been reported for the exudate gum of A. saman and the seed of M. oleífera. Nevertheless something to highlight is that the coagulation efficiency in raw water of extracts prepared from stems of H. cf. trigonus, and bark of G. ulmifolia and M. oleífera have not been investigated. Plant samples were taxonomically classified in the Herbarium of the University of Cordoba –HUC. Table 1 shows the type of plant material used and its origin.
2.3. Preparation of coagulant extracts
The epidermis of H. cf. trigonus stems collected was removed, cut into small pieces of about 1 cm2 and dried in a K Gemmy hot air sterilizer, model YCO-010 at 103°C during 2 hours [19, 20]. The A. saman exudate gum was collected one week later after making grooves shaped cuts in the trunk of the selected trees, the gum was stored in glass recipients and dried in the oven at 45°C during 8 hours [21, 22]. M. oleífera seed was obtained from the dried pods that were manually removed from the shell and then dried at room temperature through 1 day [16, 23]. Once dried, gum, stems and seeds were ground in a manual grinder of the brand Corona, obtaining a fine powder that was sieved using a mesh of 0.6 mm of the brand Grain Test (Number 30 according series of Tyler ATSM E-11/2004). The M. oleífera bark and G. ulmifolia were crushed with a hand grater for obtaining small particles [24].
Then, 10.0 grams of each of the five processed plant materials were taken and dissolved up to 1.0 liter with 1.0% (w/v) saline solution. The solutions were initially mixed through 1 hour with a Schott E & Q AMPC-1 magnetic stirrer, then centrifuged at 3,500 rpm during 10 minutes in a centrifuge of the brand K Gemmy model PLC-05 and finally filtered under reduced pressure with a vacuum GAST-Mod-DUAp104-AA using a cellulose filter paper. The filtrates were labeled as coagulants saline extracts (SCE 10,000 mg/L) for each processed plant material and kept refrigerated at 4°C.
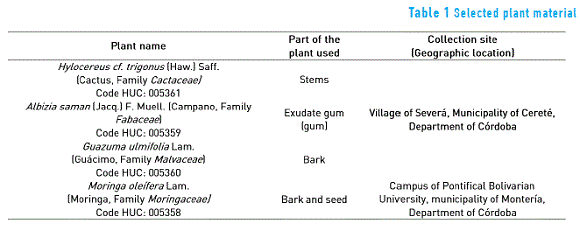
Total carbohydrates were quantified for each of the five SEC by the Anthrone method (absorbance at λ= 625 nm), using a standard starch solution (Starch, SIGMA-ALDRICH, CAS: 9005 -25 to 8, 33615-250G, Lot # SBZC3340V). Moreover, the protein content was determined by the Biuret method (absorbance at λ= 540 nm) using a standard albumin solution from bovine serum (ABS, 30%±2% in NaCl 0.85%, SIGMA-ALDRICH, A7284, 50ml, Lot # SLBD0064V), the colorimetric measurements were made with a spectrophotometer Thermo Scientific Genesys 10S UV-Vis; also an analysis by Fourier Transform Infrared Spectrometry –FTIR using a Shimadzu IRTracer-100 was performed in order to identify the active functional groups of molecules involved in the coagulant process [9].
2.4. Jar tests
To determine the optimum coagulant dose able to remove the maximum raw water turbidity, a jar test was performed in a flocculator E&Q model F6-330-T [25-27]. SEC at dosage of 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 70, 80, 90, 100 and 200 mg/L (17 doses) were applied to raw water samples with initial turbidity of 56, 71, 104, 200 and 301 NTU (5 levels), for a total of 85 samples for each SEC. Rapid mixing process was maintained at 200 rpm up 1 minute, while the slow mixing was 40 rpm for 20 minutes. Samples were subjected to sedimentation during 20 minutes and then the residual turbidity was measured with a turbidimeter HACH 2001P following the standard methods of the American Public Health Association (APHA), 2005 [28]. The residual turbidity of sample was RTS. The same coagulation test was performed without coagulant as the blank. The residual turbidity in the blank was RTB. Coagulation activity was calculated as shown in Eq. (1) [13]:
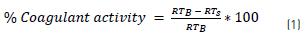
The turbidity removal percentage was calculated as a function on the initial turbidity (Ti) and residual turbidity of the sample (RTS), according to Eq. (2):
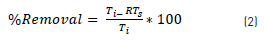
2.5. Model and statistical analysis
Experiments were performed by triplicate for each of the doses tested with the five SEC. For statistical and graphical analysis the Desing Expert 8.0.2.0 Trial version software was used. A cubic model was generated and an analysis of variance was applied in order to visualize the relationship between the experimental variables and responses through surface charts [29, 30].
3. Results and discussion
The total content of carbohydrates and proteins for the five SEC ranged from 85.08 mg/L to 1,890.30 mg/L and 0.13 mg/mL to 4.43 mg/mL, respectively, as shown in the Table 2. These compounds are mostly classified as natural coagulant [15, 16], and their concentrations give an indication of the degree of coagulation efficiency of the five SEC studied.
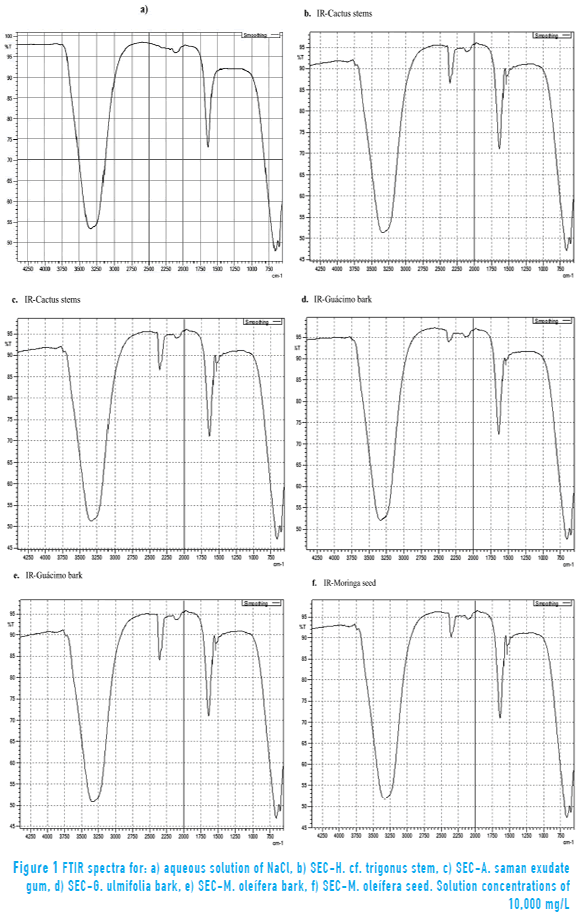
Figure 1 shows the infrared spectrum (IR) of the five SEC. Spectra qualitatively confirm the presence of functional groups since the characteristic absorption bands generated that are specific vibration patterns of those molecules.
Spectroscopic analysis of the aqueous sodium chloride solution and the five SEC exhibited the following results: The infrared spectrum (FTIR 1a, Liquid) of the aqueous sodium chloride solution showed characteristic bands at the following wavenumbers (cm-1): 3,500-4,000 (symmetric and asymmetric and tensions of O-H or free N-H); 1,600 (Flexions of N-H or O-H), typical signals of water molecule, in this case corresponds to an aqueous solution of NaCl 10,000 mg/L, and sodium chloride is transparent to the radiation in the IR region of the electromagnetic spectrum.
Infrared spectra (FTIR 1b, 1c, 1d, 1e and 1f, Liquid) of the five SEC were similar and showed the following characteristic bands (wavenumber, cm-1): at 3,500-4,000 (symmetric and asymmetric tensions of O-H or free N-H); at 1,600-1,700 (N-H or O-H flexions, indicating the presence of the carbonyl group); at 2,350 (tensions of -N=N- o -C=C=C-, associated with the presence of molecules that may contain heterocyclic rings with nitrogen heteroatoms, which suggests they correspond to basic amino acids); at 1,950-2,300 (tensions due to the presence of triple and double bonds such as nitrile, diazo, allenes, thiocyanate, ketenes); at 1,500-1,550 showing two bands of varying intensity that may correspond to a secondary amide.
According to what was observed in the spectra, extracts would be composed of molecules with several functional groups since they were not purified and consequently were no selective to a particular substance. The FTIR spectra exhibited a similar pattern, which confirms that the extracts had similar functional groups and composition; therefore the coagulation mechanisms and /or the coagulant activity could be attributed to common molecules or substances such as proteins, carbohydrates (hetero-saccharides) and polyphenols, among others. This is in accordance with the presence of primary and secondary metabolites detected in SEC.
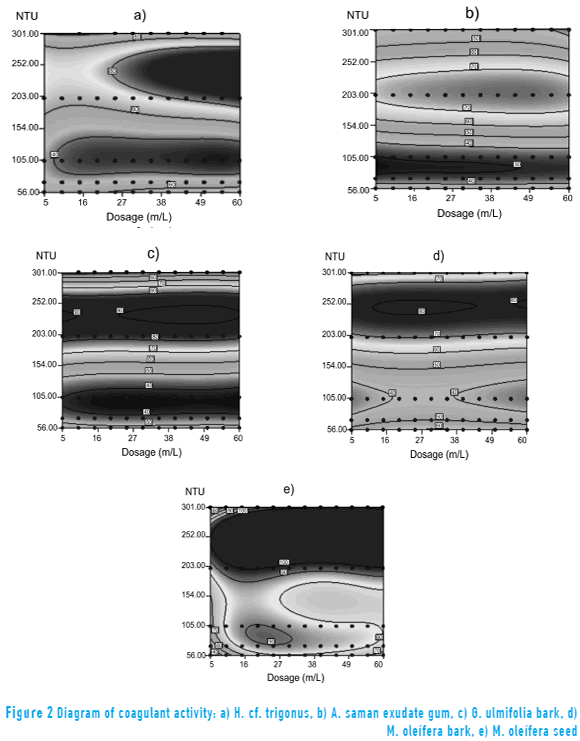
With the results of the jar test, a cubic response surface model was constructed and an analysis of variance was applied. All ANOVAs showed p-values lower than 0.05, indicating that the model terms were statistically significant. The adjusted determination coefficients (R2) were consistent for every SEC and are indicated as follows: H. cf. trigonus = 0.95; exudate gum of A. saman = 0.97; G. ulmifolia cortex = 0.95; M. oleífera bark and seed, 0.89 and 0.84 respectively. Those results suggest that the used model properly describes the coagulant activity efficiency of the tested extracts and present them as potential natural coagulants. In the Figure 2, the diagrams of surface response models are shown.
Figures 2a and 2b show diagrams for coagulant activity for extracts of H. cf. trigonus and A. saman, a similar function of the initial turbidity and the applied dose is observed in both of them. For the Sinú river raw water turbidity, which is until 100 NTU, it was possible a maximum coagulant activity for both extracts of 40 %. However, when the raw water initial turbidity was higher than 150 NTU the coagulant activity increased in a 80%, in a proportional way with the increase of the applied doses, in particular, with the gummy exudate of Campano, giving as a result flocs with gummy appearance that were formed from the extract own characteristics [9]. For both extracts, dosages higher than 60 mg/L did not have a significative coagulant activity on the samples, even that, they altered the initial physicochemical characteristics, as the increase of residual turbidity and color. These results are according to the literature [21, 22, 31], for the exudate gum of Samanea saman, Cedrela odorata and Acacia siamea, respectively. These are water soluble polymers with clarified properties, where the increase of the coagulant extract doses leads to the descent of turbidity removal and the color increase, fact that can be explained by the flocs reinvestment and colloidal particles regeneration [21]. Accordingly, with a low coagulation it can be obtained an increase in turbidity, so it is advisable to apply low dosages of these extracts, from 10 to 50 mg/L, for levels of initial turbidity of raw water from the Sinú river between 50 and 300 NTU.
The coagulant activity behavior for Cactáceas can be explained by its coagulant mechanism (adsorption and bridge between particles) where the coagulant and particles form bonds, absorbing pollutants until the saturation and the inactivation of coagulant activity [8, 15, 19, 20, 32, 33].
Mucilage in some types of Cactus contains carbohydrates, such as L-arabinosa, D-galactose, L-ramnosa, D-xilosa and galacturonic acid [15]. [32] informed that the galacturonic acid is the active ingredient that offers the coagulant capacity acting predominantly through a transition mechanism of coagulation, where the solution particles do not get in contact between them, but are linked to a polymeric material that is generated from the cactus species. The presence of carbohydrates as polysaccharides, glucose, xylose, galactose, arabinose, cactus pectin constituents, would be related to their viscous consistency (mucilage) and these substances in aqueous solution generate the suspension of other insoluble substances that induce the colloidal particles coagulation. The presence of long chain polymers on SEC-Cactus may be related to the elongated appearance of flocs. The SEC of A. saman and H. cf. trigonus, contain levels of total carbohydrates from 1,341.20 mg/L to 163.36 mg/L and total protein from 1.76 mg/mL to 0.35 mg/mL, respectively. Coagulants extracts with high values of carbohydrates and appreciable amounts of protein, provide molecules that may be involved in coagulation mechanisms and thus, in the turbidity removal.
In Figures 2c and 2d the efficiency of coagulant activity of G. ulmifolia bark and M. oleífera is shown, a similar behavior between them can be appreciated. For a turbidity higher than 200 NTU, a better efficiency was obtained between 70% and 80%, for a turbidity less than 100 NTU the efficiency of coagulant activity were less than 50%, independent of the applied doses. This behavior can be related to the bark similar composition, to the tannins presence or other phenolic composites extracted from these cortices, whose efficacy as a natural coagulant for water treatment is influenced by its chemical structure and modification grade, providing an indication that the phenolic groups are available on the possible structure for molecular interactions with charged particles in solution are given, making them more effective in their coagulant ability [15, 34]. Such as the SEC of Cactus, Guácimo extract also generates a viscous substance which precipitates in alcohol and has coagulant properties, it is known that it has been used effectively in the cane juice clarification [24, 35] and consistent with this research results, is also efficient in turbidity removal of raw water. The SEC of G. ulmifolia bark and M. oleífera, contain levels of total carbohydrates of 61.00 mg/L and 85.08 mg/L and total protein 0.13 mg/mL and 0.18 mg/mL respectively. These compounds have been reported as potential coagulants such as the phenolic compounds contained in the trees bark. From these results, it is recommended to apply doses of the extracts from M. oleífera bark and G. ulmifolia bark between 5 and 30 mg/L for turbidity initial levels of raw water of the Sinú river between 50 and 300 NTU.
The coagulation mechanism of adsorption and bridge between particles related to extracts of Cactus, Campano and barks of Guácimo and Moringa, occurs due to chemical forces, where interactions between the surface of the colloidal particles and coagulant are established by means of covalent bonds, ionic bonds, hydrogen bonds, coordinate bonds, intermolecular attraction forces or van der Waals forces. Therefore, with more sites adsorption available in the coagulant, greater amounts of colloidal molecules are absorbed. Polymeric high molecular weight molecules can be chemically adsorbed on the colloidal particles and each branch of the polymer can be adsorbed by another colloid, providing molecular bridges, binding the particles and forming a floc, destabilizing the colloidal particles and inducing deposition [15].
Figure 2e shows that for M. oleífera seed coagulant activity range is very wide, with maximum values of 90% for turbidity between 50-100 NTU. However, coagulant activity increases up to values of 98% for initial turbidity levels greater than 200 NTU across the range of doses applied. A similar behavior was found in the bark of M. oleífera, so it is assumed that they would have coagulant compounds in common.
In contrast to the others SEC tested, the M. oleífera seed showed a typical behavior of coagulation by adsorption and charge neutralization [36]. The efficiency of the clotting activity was dependent on the applied dose and increased proportionally with the level of turbidity. [15] suggests that the active cationic coagulants of M. oleífera are globular proteins soluble in water and in saline with average molecular weight of 8.5 kDa and about 65 amino acids with -R ionizable group. Protein of M. oleífera provides sites than can absorb and neutralize negatively charged impurity particles, induce the coagulation and increase the removal of turbidity. It is expected that the protein has a secondary structure, with little steric hindrance, so that the interactions can be easier with the colloids, that will make it a more efficient coagulant. Total carbohydrate content and total protein for the SEC of M. oleífera seed, was 1,980.30 mg/L and 4.43 mg/mL, respectively, greater than those found for the other four SEC amounts, concentrations supporting the presence of said active protein in the extract applied and contributed greatly to the increase in coagulant activity. Consistent with the results achieved by [37] for raw water collected from a treatment plant reservoir stocked by the Pueblo Viejo in the state of Zulia, Venezuela, the efficiency of the seeds of M. oleífera was very appreciable to remove turbidity between 80.1% and 94.3%, showing greater efficiency at higher turbidity values. Similarly, the literature [23] reported for natural waters with low turbidity from shallow wells in Meanwood Yorkshire Water and River Valley, UK, show turbidity removal up to 76% when using M. oleífera seeds. In addition to the above, it is effective to apply doses of seed extract of M. oleífera between 10 and 45 mg/L for initial levels of raw water turbidity Sinú river from 50 NTU to 300 NTU.
In all samples tested, no significant changes in pH and alkalinity after application of the doses of the five natural coagulants occurred.
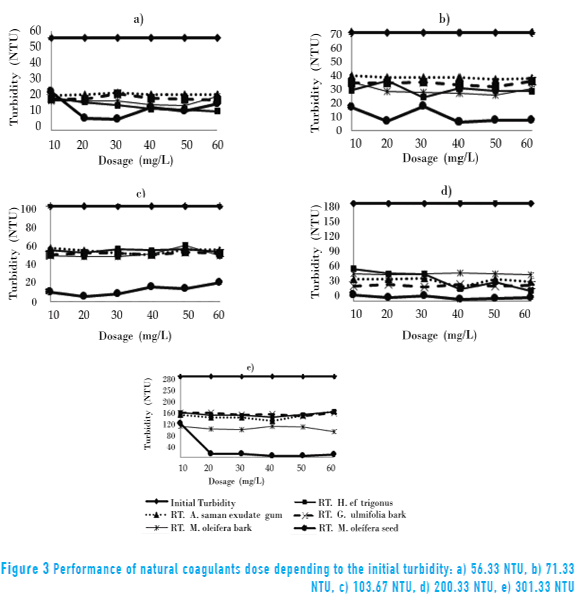
In Figure 3, the behavior of each extract tested in terms of the initial turbidity of raw water compared Sinú river.
The behavior of turbidity removal for H. cf. trigonus, exudate gum of A. saman, bark of G. ulmifolia and M. oleífera was very similar to each other, unlike what was found for M. oleífera seed.
Figures 3a and 3b show that the removal efficiency of the SEC was directly proportional to turbidity, to lower initial levels of 100 NTU, except for studies with M. oleífera seed. However, for larger values, it was independently applied as the initial turbidity of raw water dose. To initial turbidity of 100 NTU and 200 NTU, the residual turbidity ranged from 40 NTU to 60 NTU independent of dose and the type of coagulant (H. cf. trigonus, A. saman exudate gum, barks of G. ulmifolia and M. oleífera), with removal percentages between 50% and 90% (Figures 3c and 3d). For high turbidity of Sinú river's raw water (> 200 NTU, Figure 3e) turbidity residuals higher than 120 NTU were obtained, making it non-viable to use these coagulants in water treatment process, which, according to the Colombian norm, the maximum acceptable value is 2.0 NTU [38]. These extracts showed constants values the residual turbidity, independently from the doses, perhaps because they have coagulation mechanisms in common, generally associated with charge neutralization and adsorption, and bond between particles, which greatly increase the number available adsorption sites with respect to the chemical structures of natural coagulants [15]. M. oleífera extract had a very different behavior to the other natural coagulants, being more efficient in terms of increased turbidity and varying initial dose achieving turbidity removal percentages up to 99%.
4. Conclusion
The five saline extracts of natural coagulants showed removal efficiencies that prevent achieving the level of residual turbidity requirements of the quality standard for drinking water of Colombia (2 NTU). However, with a sedimentation process and supplementary filtration, it can be used as alternative coagulation-flocculation water treatment systems valid for small scale or in rural areas. The SEC from M. oleífera seed reached 4 unities of residual turbidity with optimal doses and in samples with high turbidity, having the best result with a coagulant activity until 98%, followed by the SEC of H. cf. trigonus stem, 90%, then by the SEC of G. ulmifolia bark and A. saman exudate gum, 83%, and finally the SEC from M. oleífera bark with 77% of removal.
According to coagulation mechanisms of SEC for barks G. ulmifolia and M. oleífera, the removal efficiency did not depend on the initial turbidity of raw water from the Sinú river or the dosages applied, while for the SEC M. oleífera seed, stems cf. H. trigonus gum exudate and A. saman, if dependent on these variables.
5. Acknowledgements
Thank the University of Córdoba and the Pontifical Bolivarian University for their valuable support, to Sixto Bermúdez and Roberto Pérez for their help and guidance in this project.
6. References
1. S. Yan, K. Nagendra, T. Yeong, M. Eshwaraiah, R. Nagasundara. "Utilization of plant-based natural coagulants as future alternatives towards sustainable water clarification". Journal of environmental sciences. Vol. 26. 2014. pp. 2178-2189. [ Links ]
2. United Nations International Children's Emergency Fund (UNICEF). Soap, toilets and taps: A foundation for healthy children. UNICEF. 2009. Available on: http://www.unicef.org/wash/files/FINAL_Soap_Toilets_Taps.pdf. Accessed: September 17, 2013. [ Links ]
3.& M. Pritchard, T. Mkandawire, A. Edmondson, J. O'Neill, G. Kululanga. "Potential of using plant extracts for purification of shallow well water in Malawi". Physics and Chemistry of the Earth. Vol. 34. 2009. pp. 799-805. [ Links ]
4. World Health Organization (WHO). Agua, saneamiento y salud (ASS). 2012. Available on: http://www.who.int/water_sanitation_health/monitoring/es/. Accessed: September 18, 2013. [ Links ]
5. Ministerio de Ambiente, Vivienda y Desarrollo Territorial (MAVDT). Título J. Alternativas Tecnológicas en Agua y Saneamiento para el Sector Rural. Reglamento Técnico del Sector de Agua Potable y Saneamiento Básico, MAVDT. Bogotá, Colombia. 2010. pp. 1-284. [ Links ]
6. Banco Interamericano de Desarrollo (BID). Programa de abastecimiento de agua y saneamiento en zonas rurales de Colombia. Informe de Gestión Ambiental y Social (IGAS), BID. 2011. Available on: idbdocs.iadb.org/wsdocs/getDocument.aspx?DOCNUM=36733656. Accessed: August 1, 2014. [ Links ]
7. Z. Abidin, N. Shamsudin, N. Madehi, S. Sobri. "Optimisation of a method to extract the active coagulant agent from Jatropha curcas seeds for use in turbidity removal". Industrial Crops and Products. Vol. 41. 2013. pp. 319-323. [ Links ]
8. A. Villabona, I. Paz, J. Martínez. "Caracterización de la Opuntia ficus-indica para su uso como coagulante natural". Rev. Colomb. Biotecnol. Vol. 15. 2013. pp. 137-144. [ Links ]
9. W. Subramonian, T. Yeong, S. Chai. "A comprehensive study on coagulant performance and floc characterization of natural Cassia obtusifolia seed gum in treatment of raw pulp and paper mill effluent". Industrial Crops and Products. Vol. 61. 2014. pp. 317-324. [ Links ]
10. L. Guzmán, A. Villabona, C. Tejada, R. García. "Reducción de la turbidez del agua usando coagulantes naturales: una revisión". Rev. U.D.C.A Act. & Div. Cient. Vol. 16. 2013. pp. 253-262. [ Links ]
11. S. Bondy. "The neurotoxicity of environmental aluminum is still an issue". Neuro Toxicology. Vol. 31. 2010. pp. 575-581. [ Links ]
12. T. Peder. "Aluminium as a risk factor in Alzheimer's disease, with emphasis on drinking water". Brain Research Bulletin. Vol. 55. 2001. pp. 187-196. [ Links ]
13. M. Antov, M. Sciban, J. Prodanovic. "Evaluation of the efficiency of natural coagulant obtained by ultrafiltration of common bean seed extract in water turbidity removal". Ecological Engineering. Vol. 49. 2012. pp. 48-52. [ Links ]
14. T. Okuda, A. Baes, W. Nishijima, M. Okada. "Isolation and characterization of coagulant extracted from Moringa oleifera seed by salt solution". Wat. Res. Vol. 35. 2001. pp. 405-410. [ Links ]
15. C. Yin. "Emerging usage of plant-based coagulants for water and wastewater treatment". Process Biochemistry. Vol. 45. 2010. pp. 1437-1444. [ Links ]
16. H. Bhuptawat, G. Folkard, S. Chaudhari. "Innovative physico-chemical treatment of wastewater incorporating Moringa oleifera seed coagulant". Journal of Hazardous Materials. Vol. 142. 2007. pp. 477-482. [ Links ]
17. J. Arboleda. Teoría y práctica de la purificación del agua. 3rd ed. Ed. Mac-Graw Hill. Bogotá, Colombia. 2000. pp. 1-836. [ Links ]
18. J. Feria. Rio Sinú, Colombia: Modelización calidad del agua y metales pesados en los sedimentos. 1st ed. Ed. Académica Española. Saarbrücken, Germany. 2012. pp. 1-156. [ Links ]
19. Y. Parra, M. Cedeño, M. García, I. Mendoza, Y. González, L. Fuentes. "Clarificación de aguas de alta turbidez empleando el mucílago de Opuntia wentiana (Britton & Rose) / (Cactaceae)". Redieluz. Vol. 1. 2011. pp. 27-33. [ Links ]
20. D. Martínez, M. Chávez, A. Díaz, E. Chacín, N. Fernández. "Eficiencia del Cactus lefaria para su uso como coagulante en la clarificación de aguas". Rev. Téc. Ing. Univ. Zulia. Vol. 26. 2003. pp. 27-33. [ Links ]
21. G. González, M. Chávez, D. Mejías, M. Mas, N. Fernández, G. León. "Uso del exudado gomoso producido por Samanea saman en la potabilización de las aguas". Rev. Téc. Ing. Univ. Zulia. Vol. 29. 2006. pp. 14-22. [ Links ]
22. D. Mejías, M. Delgado, M. Rubi, E. Ramos, N. Acosta. "Uso potencial del exudado gomoso de Cedrela odorata como agente coagulante para el tratamiento de las aguas destinadas a consumo humano". Revista Forestal Venezolana. Vol. 54. 2010. pp. 147-153. [ Links ]
23. M. Pritchard, T. Craven, T. Mkandawire, A. Edmondson, J. O'Neill. "A comparison between Moringa oleifera and chemical coagulants in the purification of drinking water – An alternative sustainable solution for developing countries". Physics and Chemistry of the Earth. Vol. 35. 2010. pp. 798-805. [ Links ]
24. C. Ortiz, D. Solano, H. Villada, S. Mosquera, R. Velasco. "Extracción y secado de floculantes naturales usados en la clarificación de jugos de caña". Biotecnología en el Sector Agropecuario y Agroindustrial. Vol. 9. 2011. pp. 32-40. [ Links ]
25. American Society for Testing and Materials International (ASTM). Standard Practice for Coagulation-Flocculation Jar Test of Water. Standard ASTM D2035-08, ASTM International. West Conshohocken, USA. 2008. pp. 1-4. [ Links ]
26. Instituto Colombiano de Normas Técnicas y Certificación (ICONTEC International). Procedimiento para el ensayo de coagulación-floculación en un recipiente con agua o método de jarras. NTC 3903, ICONTEC International. Bogotá, Colombia. 2010. pp. 1-4. [ Links ]
27. Ministerio de Desarrollo Económico. Sección II. Título C. Sistemas de potabilización. Reglamento Técnico del Sector de Agua Potable y Saneamiento Básico, Ministerio de Desarrollo Económico. Bogotá, Colombia. 2000. pp. 1-182. [ Links ]
28. American Public Health Association (APHA). Standard methods for examination of water and wastewater. 21st ed. Ed. Centennial. Washington, USA. 2005. pp. 1-11. [ Links ]
29. S. Shamsnejati, N. Chaibakhsh, A. Reza, S. Hayeripour. "Mucilaginous seed of Ocimum basilicum as a natural coagulant for textile wastewater treatment". Industrial Crops and Products. Vol. 69. 2015. pp. 40-47. [ Links ]
30. S. Bathia, Z. Othman, A. Ahmad. "Coagulation-flocculation process for POME treatment using Moringa oleífera sedes extrat: Optimization studies". Chemical Engineering Journal. Vol. 133. 2007. pp. 205-212. [ Links ]
31. A. Fernández, M. Chávez, F. Herrera, M. Mas, D. Mejías, A. Díaz. "Evaluación del exudado gomoso de Acacia siamea como coagulante en la clarificación de las aguas para consumo humano". Rev. Téc. Ing. Univ. Zulia. Vol. 31. 2008. pp. 32-40. [ Links ]
32. S. Miller, E. Fugate, V. Craver, J. Smith, J. Zimmerman. "Toward understanding the efficacy and mechanism of Opuntia spp. as a natural coagulant for potential application in water treatment". Environ Sci. Technol. Vol. 42. 2008. pp. 4274-4279. [ Links ]
33. J. Zhang, F. Zhang, Y. Luo, H. Yang. "A preliminary study on cactus as coagulant in water treatment". Process Biochemistry. Vol. 41. 2006. pp. 730-733. [ Links ]
34. M. Özacar, I. Sengil. "Evaluation of tannin biopolymer as a coagulant aid for coagulation of colloidal particles". Colloids and Surfaces A. Vol. 229. 2003. pp. 85-96. [ Links ]
35. R. Villatoro, L. Luna, A. González. "El cuaulote. Recurso herbolario de Chiapas". Ciencias. n.o 83. 2006. pp. 18-26. [ Links ]
36. A. Ndabigengesere, K. Subba, B. Talbot. "Active agents and mechanism of coagulation of turbid waters using Moringa oleifera". Wat. Res. Vol. 29. 1995. pp. 703-710. [ Links ]
37. Y. Caldera, I. Mendoza, L. Briceño, J. García, L. Fuentes. "Eficiencia de las semillas de Moringa oleífera como coagulante alternativo en la potabilización del agua". Boletín del centro de investigaciones biológicas. Vol. 41. 2007. pp. 244-254. [ Links ]
38. Ministerio de la Protección Social y Ministerio de Ambiente, Vivienda y Desarrollo Territorial. Resolución número 2115 (22 jun 2007). Bogotá, Colombia. 2007. pp. 2-4. [ Links ]