Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230
Rev.fac.ing.univ. Antioquia no.77 Medellín Oct./Dec. 2015
https://doi.org/10.17533/udea.redin.n77a03
ARTÍCULO ORIGINAL
DOI: 10.17533/udea.redin.n77a03
Thermogravimetric characteristics and kinetics of pyrolysis of coal blends
Características termogravimétricas y cinética de pirolisis de mezcla de carbones
Juan Manuel Barraza-Burgos1*, Edward Andrés García-Saavedra1, Deisy Chaves-Sanchez2, María Patricia Trujillo-Uribe2, Francisco Javier Velasco-Charria1, Jaime José Acuña-Polanco1
1Grupo de Ciencia y Tecnología del Carbón, Facultad de Ingeniería, Universidad del Valle. Ciudad Universitaria Meléndez, Calle 13 # 100-00. A. A. 25360. Cali, Colombia.
2Laboratorio de Investigación en Multimedia y Visión, Facultad de Ingeniería, Universidad del Valle. Ciudad Universitaria Meléndez, Calle 13 # 100-00. A. A. 25360. Cali, Colombia.
* Corresponding author: Juan Manuel Barraza Burgos, e-mail: juan.barraza@correounivalle.edu.co
(Received July 27, 2014; accepted July 08, 2015)
ABSTRACT
Thermogravimetric characteristics and pyrolysis kinetics of coal blends were investigated using a thermogravimetric analyser under heating rate of 20°C/min, from room temperature to 850°C. Original coal samples were obtained from three Colombian regions: Valle del Cauca, South West (VAL), Antioquia, West Midlands (ANT) and Cundinamarca, Midlands (CUN). Coal blend samples (50-50 % w/w) were obtained from original coal of these three regions. The weight loss curves of coals obtained by thermogravimetry (TG) showed different trends. These may indicate that pyrolysis behaviours were not similar due to coal molecular structures have different chemical bonds. In addition, a kinetic analysis was performed to fit TG data. In the analysis, it was assumed that a single chemical reaction described the pyrolysis of coals and coal blends. A Synergy Activation Energy Ratio (SAER) was defined in order to determine an additive effect between coals and coal blends, during pyrolysis. Obtained results showed that the SAER of the CUN-VAL coal blend is close to the unit, meaning that the blend has an additive behaviour. However, the SAER of ANT-VAL coal blend is lower than one, whereas of the ANT-CUN coal blend is higher than one. It suggests that VAL and CUN coals may have a non-additive reaction when they are blended with ANT coal.
Keywords: Thermogravimetry, pyrolysis, kinetics, coal blends
RESUMEN
Las características termogravimétricas y la cinética de la pirolisis de mezclas de carbones se investigaron usando un analizador termogravimétrico con velocidades de calentamiento de 20°C/min desde temperatura ambiente hasta 850°C. Las muestras de carbones originales se obtuvieron de tres regiones colombianas: Valle del Cauca (VAL), Antioquia (ANT) y Cundinamarca (CUN). Las muestras de carbones mezclados (50-50 % p/p) se produjeron a partir de carbones originales de esas tres regiones. Las curvas de pérdida de peso de los carbones obtenidas por termogravimetría (TG) mostraron diferentes tendencias. Esas curvas podrían indicar que los comportamientos durante la pirólisis no fueron similares debido a que las estructuras moleculares de los carbones tienen diferentes enlaces químicos. Además, un análisis cinético se realizó para ajustar los datos de TG. En el análisis, se supuso que una reacción química única describe la pirólisis de carbones originales y mezcla de carbones. Una relación de Sinergia de Energía de Activación (SAER) se definió con el fin de determinar el efecto aditivo entre los carbones y la mezcla de carbones durante la pirólisis. Los resultados obtenidos mostraron que la SAER de la mezcla CUN-VAL está cerca de la unidad, lo que significa que la mezcla tiene un comportamiento aditivo. Sin embargo, el SAER de la mezcla ANT-VAL es inferior a uno, mientras que de la mezcla ANT-CUN es mayor que uno. Esto sugiere que los carbones VAL y CUN podrían tener una reacción no aditiva cuando se mezclan con el carbón ANT.
Palabras clave: Termogravimetría, pirolisis, cinética, mezclas de carbón
1. Introduction
Coal combustion is widely used at the Colombian Southwest region to generate steam process and power. However, coal production is low in this region due to the high content of ash and sulphur, which results in lower availability and higher running cost of plants as well as in environmental pollution. Local consumers import coals from the West Midlands and the Colombian Midlands having those low ash and sulphur contents, and therefore, local consumers produce blends using coals from different Colombian regions, at adequate proportions, to reach the design conditions of boilers.
Coals are usually blended to obtain better properties and behaviours during the combustion process than those of a single coal. Coals are also blended to mitigate existing problems at power stations, improve boiler performance, meet emission limits, and reduce costs. Moreover, the blended coal does not necessarily produce the same behaviour during combustion, despite complying with the design conditions of the boiler. Interactions may occur between coal components, which may not always be beneficial to the combustion. Thus, the compatibility of the alternate coal with respect to the combustion performance has to be properly evaluated [1 , 2] .
A rapid pyrolysis occurs during the coal combustion process. Gases, volatile material and non-volatile tar products are produced and a solid called char is formed [3] . Since the pyrolysis is a fundamental step in the combustion processes, the kinetics has to be understood in the adaptation of coal blends as raw material of a boiler. Understanding the kinetics of a thermal decomposition of fuels or blended fuels is crucial for designing and operating conversion systems [4] .
Thermogravimetric Analysis (TGA) is commonly used to investigate thermal events and kinetics during the pyrolysis of solid raw materials, such as coal and coal blends, biomass and plastic waste [5 - 7] . It provides a measurement of weight loss of a sample as a function of time and temperature. The kinetics of thermal events has been evaluated by the application of the Arrhenius equation corresponding to the separate slopes of constant mass degradation in each thermal event with different reaction order, activation energy, and frequency factor [8] .
Several authors commonly approximate the overall process as a first-order decomposition occurring uniformly throughout coal particles [9 - 13] . However, a simple first-order model may be inadequate for coal pyrolysis. It may be better described as a series of consecutive or parallel first-order/nth-order processes occurring at different time and temperature intervals [14 , 15] . Differences in the kinetic parameters are related to experimental methods, operating conditions, data analysis, and chemical composition of the raw materials used.
In the present study, thermogravimetric characteristics and pyrolysis kinetics of coal blends were determined by TGA at non-isothermal condition in a thermobalance reactor using a first-order decomposition. The Synergy Activation Energy Ratio (SAER) was defined in order to determine an additive effect between coals and coal blends, during pyrolysis. The pyrolysis reactivity, activation energy, and the SAER of coals and coal blends were determined.
2. Experimental
2.1. Materials
Coal samples were obtained from three Colombian regions: Valle del Cauca, Southwest (VAL), the Antioquia, West midlands (ANT) and Cundinamarca, Midlands (CUN); these samples are called coals. After pulverising coal samples, particles having less than 250 µm sizes were used. At this size, gravity has minimal influence on particles, leading to better fluidisation in a reactor and avoiding heat and mass transfer limitations [16] . Coals were mixed 50-50% w/w proportions from samples of two regions to obtain blends, which were according to the used proportion at the enterprise; these samples are called coal blends.
2.2. Pyrolysis
Pyrolysis characteristics of coals and coal blends at a non-isothermal condition were determined using a TA instrument, model SDTQ600V20.9 build2.0, under nitrogen flow of 100 ml/min. Each sample (15–20mg) was heated from room temperature to 900◦C with a heating rate of 20◦C/min.
The weight loss (TG) and the weight loss rate (DTG), as a function of temperature, were obtained continuously under dynamic conditions. The weight loss rate was calculated using the Eq. (1) [17] :
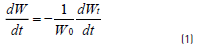
The pyrolysis conversion, x, was calculated using Eq. (2):
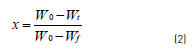
Where, W0 is the original mass of the test sample, Wtis the mass at time t and Wf is final mass at the end of pyrolysis.
3. Results and discussion
3.1. Blend coal characteristics
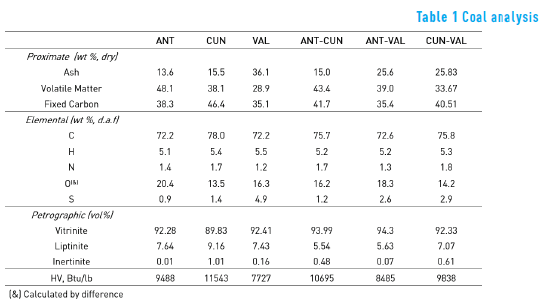
The proximate, the ultimate, the petrographic and the calorific values of each coal sample are shown in Table 1. It can be observed that the VAL coal contains the highest ash content whereas the ANT coal contains the lowest ash content. The CUN coal presented the highest fixed carbon value. Those results are in agreement with the carbon content and heat value, where the CUN coal showed the highest values. Note that the VAL coal presented the highest sulphur content. In regard to the petrographic analysis, unblended and blended coals showed large quantities of vitrinite and small concentration of inertinite macerals.
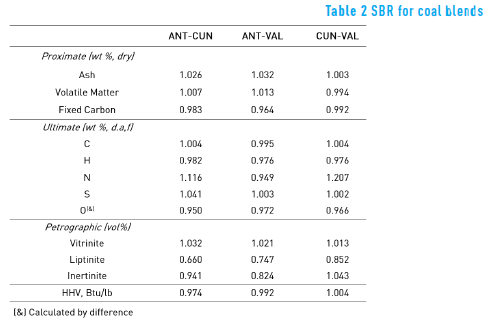
Table 1 also shows the proximate, the ultimate, the petrographic and the calorific values of each blended sample. In order to evaluate any additivity effect between coal and coal blends, this work defined the parameter Synergy Blend Ratio (SBR), which has not been previously shown, according to Eq. (3):
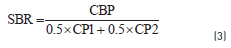
Where CBP is the Coal Blend Property, (i.e ash and fixed carbon) and CP1 and CP2 are the Coal Property of individual coal samples – coal sample 1 and coal sample 2.
If SBR> 1, it means that the CBP is higher than the expected property ( 0.5XCP1 + 0.5XCP2) . In the case that SBR = 1, the CBP is equal to the expected property, meaning that there is an additive effect between properties of samples coal 1 and 2, whereas if SBR< 1, the CBP is lower than the expected property.
The SBR values for each coal blend are shown in Table 2. As it can be seen, in general, the SBR values are around one, which means that most of the properties have an additive behaviour. However, Nitrogen (N) presented, in all cases, SBR values far from one (higher than 5% relative to SBR = 1).
The highest value of SBR, for N element, was obtained with the CUN-VAL blend, whereas the lowest one was obtained with the ANT-VAL blend. These values suggest that the VAL coal may produce the synergistic effect. In relation to SBR, in the petrographic analysis, the vitrinite showed an additive effect. However, liptinite and inertinite presented deviation from additivity, which may be due to changes – mainly in liptinite (the softness) – during grinding process.
3.2. Pyrolysis of coal and coal blends
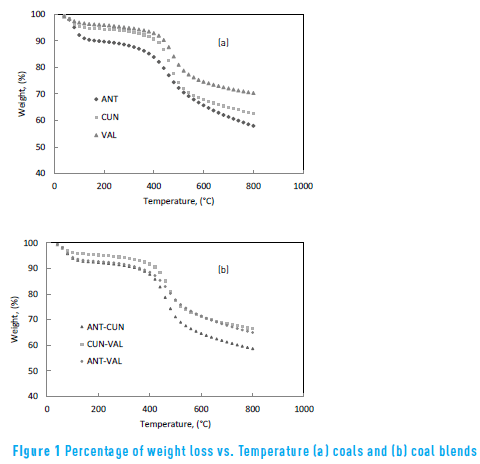
The TG curves of coals and coal blends in non-isothermal condition are shown in Figure 1. It can be observed that the weight loss increased when temperature increased, for all samples.
The weight loss curves of coals have different trends, which indicate that different pyrolysis behaviours occur due to the different chemical bonds and molecular structures of the coals. The obtained weight loss results are contrary to the results found in other work [17] using plastic samples, who determined that the weight loss curves of some plastics have almost the same trends, indicating that they have the same pyrolysis behaviours due to similar chemical bonds in their molecular structures. Therefore, differences of the structures between coal and plastic samples could explain this behaviour. In general at a temperature higher than 75 °C, the ANT coal presented the highest weight loss, whereas the VAL coal showed the lowest. These can be related to the ANT coal, because it contains high content of volatile matter, whilst the VAL coal contains the lowest.
Regarding coal blends, the weight loss curves lie between the weight loss curves of parent coals. Those results are in agreement with other work [2] , who found that the TG curves for the blended coals are between those for the parent coals, including a weight loss due to moisture evaporation and oxygen chemisorptions. The lowest final weight loss is the ANT-CUN blend. Relating with the proximate results in Table 1, the ANT-CUN blend has the highest content of volatile matter and lowest ash content.
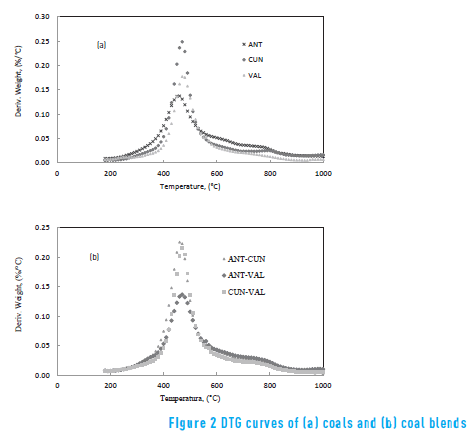
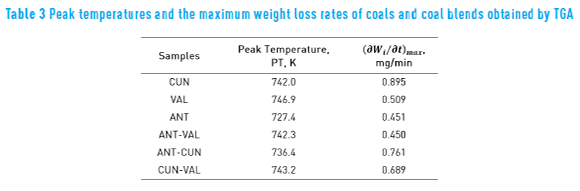
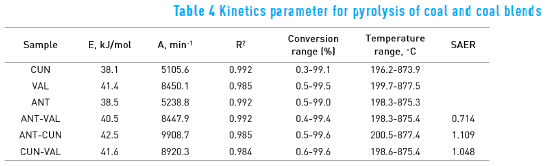
Figure 2 shows the DTG curves of the coal and the coal blends. Peak temperatures (TP) and maximum weight loss rates (∂Wi ⁄∂t )max– are some characteristic parameters obtained from pyrolysis thermogravimetric data – which are shown in Table 3. Usually TP is related to material structure [4] and gives information about pyrolysis reactivity of each coal.
In Table 3, the ANT coal presented both the lowest peak temperature and the highest maximum weight loss rate. It means that the ANT coal has the highest pyrolysis reactivity, which may be due to high volatile matter content. Regarding coal blends, the ANT-CUN showed the lowest peak temperature and the highest maximum weight loss rate. The ANT and the CUN coals contain high volatile matter, which is in agreement with the result of the blend.
A comparison between the pyrolysis behaviour of coal and coal blends, in Figure 2, shows that the CUN coal overlaps the evolution zone of volatile matter from the VAL coal, whereas the VAL coal overlaps the ANT coal. Accordingly, the thermal behaviours of each coal during pyrolysis differ from each other. Such different thermal behaviour is due to the different chemical composition of the volatile matter released during the pyrolysis of those coals. Similar results were found in other work [18] .
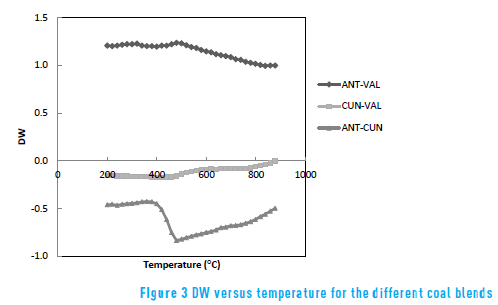
The difference of weight loss (DW) is defined in order to investigate the synergistic effect between the coal samples [16 , 17] . Taking DW as a function of the synergistic effect of each material during pyrolysis, DW = Wblend - (x1W1 + x2W2) where Wblend is the weight loss of blend, x1 and x2 are the weight fractions of each material in the blend (0.5), W1 and W2 are the weight losses of each material in the same operational conditions. Figure 3 shows DWversus temperature for the different coal blends.
It can be observed that DW is larger than one for all temperature using the ANT-VAL blend; it may indicate that there is not any synergistic effect. DW is negative for all temperature using the CUN-VAL blend, with decreasing behaviour in the range 200°C- 500°C and increase behaviour after 500°C.
3.3. Kinetics analysis
Activation energy (E), frequency factor (A), and reaction model function f(x) are the parameters that characterise the kinetics reaction of a carbonaceous solid. Those parameters are usually found in a non-isothermal condition with a constant heating rate to determine the decomposition of each fuel component at a given temperature range.
The pyrolysis reaction for coal and coal blends are expressed by the Arrhenius equation, Eq. (4):
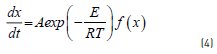
Where x is carbon conversion of fuel, t is time and k is reaction rate constant. Using a non-isothermal condition, Eq. (2) with a constant heating rate, i.e.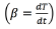 becomes Eq. (5):
becomes Eq. (5):
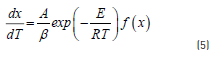
The Eq. (5) considers a first order reaction model, f(x) = (1 - x) , as reported in other work [19] .
Using a first order kinetic model and an integral method [20] Eq. (5) is transformed into Eq. (6) [16] :
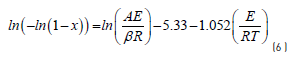
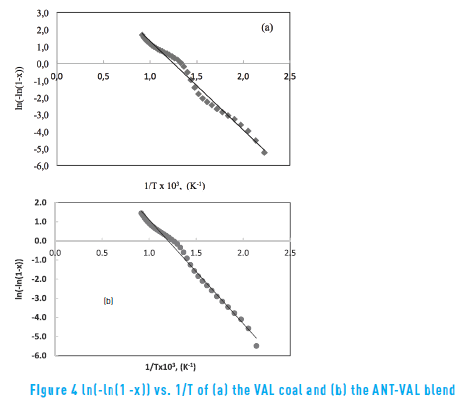
The activation energy (A) can be obtained from Eq. (6) when the left side In(-In(1 - X)) is plotted vs. 1/T . The slope of the straight line is the activation energy (E) and the intercept represents the frequency factor (A). In this research, a single chemical reaction is described during pyrolysis of coal and coal blends, that is in agreement with the results of other work [16] , using 100% coal and found an activation energy of 52.5 KJ/mol at a temperature range of 182-894 C.
Figure 4 shows the plots of In(-In(1 - X)) vs. 1/T, for the VAL coal and the ANT-VAL blend. In general, the pyrolysis process can be described by a first-order reaction. Table 4 shows the obtained kinetic parameters of coal and coal blends in the non-isothermal condition, obtained determination coefficients (R2) over 0.984. Therefore, the first order reaction at the given temperature range fits the experimental data by a linear relation.
Table 4 indicates that activation energy and pre-exponential factor of the coal blends have not additive behaviour. This suggests that the pyrolysis mechanisms of coal blends are different from those coals.
In general, coals and coal blends were pyrolyzed at the entire temperature range with the activation energy in the range of 40.1 to 43.8 kJ/mol, in agreement with the parameters found in the literature [21] . It is noteworthy that the conversion is close to 100% thorough all the temperature range for all, coals and coal blends. It also indicates that the coals were decomposed over all the temperature range used.
In order to determine any additive effect in coal blends during the pyrolysis, a Synergy Activation Energy Ratio (SAER) is defined by the Eq. (7):
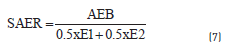
Where, AEB is the Activation Energy of the Coal Blend and E1and E2 are the Activation Energy of each coal in the blend. If SAER> 1, it means that the activation energy of the blend is higher than the expected activation energy (0.5 x E1 + 0.5 x E2) . In the case that SAER = 1, the activation energy of the blend is equal to the expected activation energy, whereas if SAER<1, the activation energy of the blend is lower than the expected activation energy.
The SAER values are also shown in the seventh column of Table 4. The CUN-VAL blend has the SAER value close to one that means an additive behaviour of the activation energy. However, the SAER value of the ANT-VAL coal blend is lower than one, whereas the SAER value of the ANT-CUN is higher than one, indicating an interaction effect when ANT coal is blended with VAL and CUN coals. The VAL coal has the highest ash content, which degraded the liberation of volatile matter of the ANT coal, whereas the CUN coal has similar ash content than the ANT coal improving the devolatilization of the ANT coal.
4. Conclusions
From the TGA analysis of the coal and blend coal the results were:
· The ANT coal presented the highest devolatilization rate, whereas the VAL coal showed the lowest. It means that the ANT coal has the highest pyrolysis reactivity, which may be due to high volatile matter content. Regarding coal blends, the weight loss curves lie between the weight loss curves of coals. The lowest final weight loss is the ANT-CUN blend.
·The parameter DW is larger than one for all temperature using the ANT-VAL blend, it may indicate that there is not any synergistic effect. DW is negative for all temperature using the CUN-VAL blend, with decreasing behaviour in the range 200°C- 500°C and increase behaviour after 500°C.
From the results of the kinetics pyrolysis of coals and coal blends, the following conclusions may be drawn:
·A First-order kinetic model and a single chemical reaction described the pyrolysis of coal and coal blends.
·The Synergy Activation Energy Ratio (SAER) showed that an interaction effect occurs when ANT coal is blended with VAL and CUN coals.
5. Acknowledgments
This work was partially supported by the Colombian Science Institute (Colciencias) through the project ''Morfología de carbonizados: Herramienta para evaluar la reactividad de carbones en el proceso de combustión'', grant funded No. 1106-521-28510.
6. References
1. D. Vamvuka, E. Kastanaki and M. Lasithiotakis, ''Devolatilization and combustion kinetics of low-rank coal blends from dynamic measurements'', Industrial & Engineering Chemistry Research, vol. 42, no. 20, pp. 4732-4740, 2003. [ Links ]
2. C. Moon et al., ''Thermochemical and combustion behaviors of coals of different ranks and their blends for pulverized-coal combustion'', Applied Thermal Engineering, vol. 54, no. 1, pp. 111-119, 2013. [ Links ]
3. D. Vargas, D. Chaves, M. Trujillo, J. Pineres and J. Barraza, ''Beneficiated coals' char morphology'', Ingeniería e Investigación, vol. 33, no. 1, pp. 13-17, 2013. [ Links ]
4. Q. Liu, H. Hu, Q. Zhou, S. Zhu and G. Chen, ''Effect of inorganic matter on reactivity and kinetics of coal pyrolysis'', Fuel, vol. 83, no. 6, pp. 713-718, 2004. [ Links ]
5. O. Senneca, R. Chirone and P. Salatino, ''A thermogravimetric study of nonfossil solid fuels. 2. Oxidative pyrolysis and char combustion'', Energy and Fuels, vol. 16, no. 3, pp. 661-668, 2002. [ Links ]
6. M. Ishaq et al., ''Pyrolysis of some whole plastics and plastics-coal mixtures'', Energy Conversion and Management, vol. 47, no. 18-19, pp. 3216-3223, 2006. [ Links ]
7. B. Shen and Qinlei, ''Study on MSW catalytic combustion by TGA'', Energy Conversion and Management, vol. 47, no. 11-12, pp. 1429-1437, 2006. [ Links ]
8. A. Bhagavatula, G. Huffman, N. Shah and R. Honaker, ''Evaluation of thermal evolution profiles and estimation of kinetic parameters for pyrolysis of coal/corn stover blends using thermogravimetric analysis'', Journal of Fuels, vol. 2014, pp. 1-12, 2014. [ Links ]
9. M. Kök, E. Özbas, C. Hicyilmaz and O. Karacan, ''Effect of particle size on the thermal and combustion properties of coal'', Thermochimica Acta, vol. 302, no. 1-2, pp. 125-130, 1997. [ Links ]
10. M. Kök, E. Özbas, O. Karacan and C. Hicyilmaz, ''Effect of particle size on coal pyrolysis'', Journal of Analytical and Applied Pyrolysis, vol.45, no. 2, pp. 103-110, 1998. [ Links ]
11. M. Lázaro, R. Moliner and I. Suelves, ''Non-isothermal versus isothermal technique to evaluate kinetic parameters of coal pyrolysis'', Journal of Analytical and Applied Pyrolysis, vol. 47, no. 2, pp. 111-125, 1998. [ Links ]
12. N. Russell, T. Beeley, C. Man, J. Gibbins and J. Williamson, ''Development of TG measurements of intrinsic char combustion reactivity for industrial and research purposes'', Fuel Processing Technology, vol. 57, no. 2, pp. 113-130, 1998. [ Links ]
13. A. Arenillas, F. Rubiera, C. Pevida and J. Pis, ''A comparison of different methods for predicting coal devolatilisation kinetics'', Journal of Analytical and Applied Pyrolysis, vol. 58-59, pp. 685-701, 2001. [ Links ]
14. G. Puente, G. Marbán, E. Fuente and J. Pis, ''Modelling of volatile product evolution in coal pyrolysis. The role of aerial oxidation'', Journal of Analytical and Applied Pyrolysis, vol. 44, no. 2, pp. 205-218, 1998. [ Links ]
15. J. Zhou, Y. Wang, Q. Huang and J. Cai, ''Thermogravimetric characteristics and kinetic of plastic and biomass blends co-pyrolysis'', Fuel Processing Technology, vol. 87, no. 11, pp. 963-969, 2006. [ Links ]
16. M. Seo, S. Kim, S. Lee and J. Lee, ''Pyrolysis characteristics of coal and RDF blends in non-isothermal and isothermal conditions'', Journal of Analytical and Applied Pyrolysis, vol. 88, no. 2, pp. 160-167, 2010. [ Links ]
17. L. Zhou, T. Luo and Q. Huang, ''Co-pyrolysis characteristics and kinetics of coal and plastic blends'', Energy Conversion and Management, vol. 50, no. 3, pp. 705-710, 2009. [ Links ]
18. L. Vivero, C. Barriocanal, R. Álvarez and M. Díez, ''Effects of plastic wastes on coal pyrolysis behaviour and the structure of semicokes'', Journal of Analytical and Applied Pyrolysis, vol. 74, no. 1-2, pp. 327-336, 2005. [ Links ]
19. E. Biagini, F. Lippi, L. Petarca and L. Tognotti, ''Devolatilization rate of biomasses and coal–biomass blends: an experimental investigation'', Fuel, vol. 81, no. 8, pp. 1041-1050, 2002. [ Links ]
20. R. Lyon, ''An integral method of nonisothermal kinetic analysis'', Thermochimica Acta, vol. 297, no. 1-2, pp. 117-124, 1997. [ Links ]
21. S. Badzioch and P. Hawksley, ''Kinetics of thermal decomposition of pulverized coal particles'', Industrial & Engineering Chemistry Process Design and Development, vol. 9, no. 4, pp. 521-530, 1970. [ Links ]