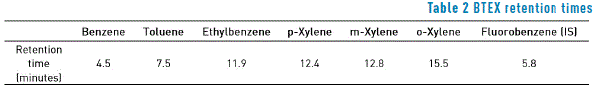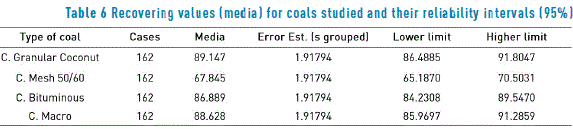Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230
Rev.fac.ing.univ. Antioquia no.79 Medellín Apr./June 2016
https://doi.org/10.17533/udea.redin.n79a13
ARTÍCULO ORIGINAL
DOI: 10.17533/udea.redin.n79a13
Validation of a methodology to determine Benzene, Toluene, Ethylbenzene, and Xylenes concentration present in the air and adsorbed in activated charcoal passive samplers by GC/FID chromatography
Validación de una metodología para la determinación de la concentración de Benceno, Tolueno, Etilbenceno y Xilenos, presentes en muestras aire y adsorbidos en captadores pasivos de carbón activado, mediante cromatografía GC/FID
Mary Luz Gallego-Díez1*, Mauricio Andrés Correa-Ochoa2, Julio César Saldarriaga-Molina2
1Facultad de Ingeniería, Universidad de Antioquia. Calle 67 # 53- 108. A. A. 1226. Medellín, Colombia.
2Grupo de Ingeniería y Gestión Ambiental (GIGA), Facultad de Ingeniería, Universidad de Antioquia. Calle 67 # 53- 108. A. A. 1226. Medellín, Colombia.
* Corresponding author: Mary Luz Gallego Díez, e-mail: mgallegodiez@gmail.com
DOI: 10.17533/udea.redin.n79a13
ISSN 0120-6230
e-ISSN 2422-2844
(Received August 28, 2015; accepted April 02, 2016)
ABSTRACT
This article shows the validation of the analytical procedure which allows determining concentrations of Benzene (B), Toluene (T), Ethylbenzene (E), and Xylenes (X) -compounds known as BTEX- present in the air and adsorbed by over activated charcoal by GC-FID using the (Fluorobenzene) internal standard addition as quantification method. In the process, reference activated charcoal was employed for validation and coconut -base granular charcoal (CGC) for the construction of passive captors used in sample taken in external places or in environmental air. CGC material was selected from its recovering capacity of BTEX, with an average of 89.1% for all analytes. In this research, BTEX presence in air samples, taken in a road of six lines and characterized for having heavy traffic, in Medellín city (Antioquia, Colombia), was analyzed. Samplers employed were located in pairs per point (in 7 transversal strips of the east, central, and west sidewalk), at heights ranging from 2.50 and 3.00 meters, at the floor level, inside a special housing for their protection. The number of total stations was twenty-one (21) in 3 kilometers, with exposition times of 28 days. Analytes desorption procedure was carried out with carbon disulfide as an extraction solvent, and in the chromatograhic analysis of gases this was performed (by triplicate) using a flame ionization detector (FID). An HP-INNOWAX chromatographic column was also used. Ultra-pure Helium, 99.99% purity, was used as carrier gas and quantification was performed (by triplicate) in the liquid extract in terms of concentration (µg/mL). The research allowed validating the methodology, obtaining recovery percentages ranging between 75.0 % and 98.2 % for all analytes, and quantification limits in µg/mL were 0.279; 0.337; 0.349; 0.391; 0.355; and 0.356 for benzene, toluene, ethylbenzene, p-xylene, m-xylene, and o-xylene, respectively, and it was proven that the validated method was a selective, specific, linear, accurate, and exact method.
Keywords: Validation, activated charcoal, volatile organic compounds (VOC's), BTEX, GC/FID, BTEX in air
RESUMEN
En este trabajo se presenta la validación del procedimiento analítico que permite determinar las concentraciones de Benceno (B), Tolueno (T), Etilbenceno (E) y Xilenos (X), compuestos conocidos como BTEX, presentes en el aire y adsorbidos sobre carbón activado, usando el método de adición de estándar interno (Fluorobenceno) para la cuantificación. En el proceso se empleó carbón activado de referencia para la validación y carbón granular (CGC) a base de coco para la construcción de los captadores pasivos, empleados en la toma de muestras en exteriores o aire ambiente. El material CGC fue seleccionado a partir de su capacidad de recuperación de BTEX, con un promedio 89,1% para todos los analitos. En la investigación se evaluó la presencia de BTEX en muestras de aire, tomadas en una vía de seis carriles y caracterizada, además, por ser de alto flujo vehicular en la ciudad de Medellín (Antioquia, Colombia). Los captadores empleados, fueron ubicados en pares por punto (en siete franjas transversales de la vía: andenes oriental, central y occidental, y a alturas que oscilaron entre los 2,50 y 3,00 metros a nivel de piso), dentro de una carcasa especial para su protección. El número de estaciones totales fue de veintiuno (21) en un trayecto de 3 km, para un total de 21 muestras recolectadas con tiempos de exposición de 28 días. El procedimiento de desorción de los analitos se realizó con disulfuro de carbono como solvente de extracción y en el análisis cromatográfico de gases se realizó (por triplicado) empleando un detector de ionización de llama (FID). Se usó, además, una columna cromatográfica HP- INNOWAX. El tiempo de corrido empleado fue de 18,5 minutos, usando Helio ultra puro, 99,99% de pureza como gas de arrastre y la cuantificación se llevó a cabo en el extracto líquido en términos de concentración (µg/mL). En la investigación se pudo validar la metodología, obteniendo porcentajes de recuperación que oscilaron entre el 75,0 y el 98,2 % para todos los analitos y los límites de cuantificación en µg/mL fueron 0,279; 0,337; 0,349; 0,391; 0,355 y 0,356; para Benceno, Tolueno, Etilbenceno, p-xileno; m-xileno y o-xileno, respectivamente y se logró demostrar que el método validado fue selectivo, específico, lineal, preciso y exacto.
Palabras clave: Validación, carbón activado, compuestos orgánicos volátiles (COV's), BTEX, GC/FID, BTEX en aire
1. Introduction
In the world, several works have established that environmental pollution is increasingly critical at urban centers, and industrial activity and vehicle traffic are defined as sources that contribute to this problem [1-3], resulting in impaired standard of living and a risk for the health of exposed populations. Based on the description above, compounds defined as BTEX (Benzene, Toluene, Ethylbenzene, and Xylenes) are a group of chemical species that are part of the well-known Volatile Organic Compounds (VOC's) frequently present in conurbated environments and included in the list of hazardous air pollutants [4]; additionally, benzene and toluene are known to be compounds with carcinogenic effects [5, 6].
Information about temporary space distribution of BTEX concentration levels has been collected at urban centers with the purpose of setting the degree of exposure and the risk level of their inhabitants. Measurement campaigns of several studies consulted exhibit disturbing results given that the concentration levels exceed the permissible limits set by regulations in relation to air quality of each country described [3, 7-11].
At populated nuclei, permanent quantification of BTEX should become one of the strategies with the highest impact for controlling levels of exposure to this type of pollutants. In Colombia, the maximum authorized levels of Benzene and Toluene in indoor or outdoor air are regulated by Resolution No. 610, March 24th, 2010, issued by the Ministry of Environment, Housing, and Territorial Development. This Resolution establishes that the annual average concentration of benzene should not exceed 5.0 µg/m3 and annual average concentration of toluene should not exceed 260.0 µg/m3 for a weekly measurement period and 1,000.0 µg/m3 for a 30-minute measurement period.
In relation to sampling methodologies, air quality has been assessed worldwide based on several techniques: automatic equipment, remote passive samplers, active samplers, and passive samplers. The advantage of automatic analyzers lies on the fact that they show data in real time and provide hour information; however, automatic analyzers become a costly and complex technique that requires personnel training. On the other hand, remote passive samplers provide data in a specific space; they are useful for measuring close to the polluting sources and their disadvantage lies on the fact that they are hard to be operated, calibrated, and validated and they are not always comparable to specific measurements. Active samplers are easily operated, affordable, and safe and require intensive work during the day and future analysis at the laboratory. Finally, passive samplers are affordable and easily operated tools, very useful for baseline studies and provide information in different time scales (weekly and monthly). In this sense, for the case of VOC's, passive samplers have become widely used devices thanks to their good performance to determine such chemical substances when they are present in urban atmospheres [3, 12].
In Colombia, few studies account for the levels of concentration of this type of (BTEX) pollutants present in the air, specifically due to two basic factors. First, monitoring networks do not operate with direct reading measurement equipment (either automatic or manual equipment), given their high cost and limited capacity to simultaneously evaluate several sites of interest. Second, local market does not provide necessary offers to supply passive measurement devices and carry out future analysis with acceptable quality criteria (laboratories with validated and/or accredited methods), just as proposed by [11].
Accordingly, an analytical method of BTEX extraction and analysis adsorbed in activated charcoal passive samplers used in air quality sampling was researched and standardized. The method was subject to a validation protocol where parameters such as selectivity, detection and quantification limits, linearity, accuracy, exactness, and recovery percentages were set for all analytes. Furthermore, the robustness parameter for Benzene, Toluene, and m-Xylene was determined. The objective of this research was to show the validation of BTEX compounds quantification methods absorbed in activated charcoal. In this sense, validation method is applied to environmental air samples, taken on a heavy traffic road in Medellín (Antioquia, Colombia).
2. Methodology
2.1. Reagents, materials, and equipment
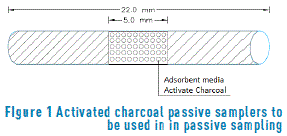
Reagents and materials used for the research included: BTEX certified standard of 2,000 µg/mL, RESTEK® brand, ALFA 99% flourobenzene internal standard; Merck® analytical-grade carbon disulfide extraction solvent; Merck® 95-97% analytical-grade sulfuric acid; Merck® 65% analytical-grade nitric acid; silica gel-60 for column chromatography (70-230 mesh); Gilian activated charcoal passive samplers, 6x70 mesh, used in the validation of laboratory chromatographic method. Coconut-base vegetal-origin activated charcoal passive captors (CGC) composed the medium used in the field and consist in glass cylinders with an internal diameter of 8.1 mm and a length of 22.0 mm (see Figure 1). Polyethylene cylinders and 4.0 mL vials with Teflon coated lid are also used. Captors were assembled inside a plastic housing which avoids the component exposition to phenomena such as invasion for birds and rain, among many others, etc.
2.2. Equipment
An HP 6890A gas chromatograph is employed with FID (Flame Ionization Detector), Agilent 6890 PLUS Software, which has an HP-INNOWAX column; 30 m x 0.5 mm and ID 0.25 um. Additionally, an analytical scale with 0.0001 g accuracy and a Vortex mixer were used.
2.3. Experimental section
Selection of extraction solvent
Extracting analytes of interest from the adsorbing material requires a solvent capacity of extracting analytes from the matrix and making them soluble. According to solubility and polarity of compounds, experiments were made with acetone, methanol, and carbon disulfide.
Purification of extraction solvent
Among the solvents employed (acetone, methanol, and carbon disulfide), that are necessary for extracting BTEX compounds of activated charcoal, It was decided to use the carbon disulfide (CS2), despite it is well known that this compound exhibits benzene traces that are eliminated by applying the OSHA method [13], which chemical principle includes a reaction of benzene nitration until reaching its purification. The CS2 purification process involves the application of OSHA methodology, which is performed with mixture with extraction hood. Such a method requires a flat-bottom flask in which 180.0 mL of compound is poured; then, 5.0 mL of concentrated sulfuric acid (H2SO4) is added, and 50 drops of concentrated nitric acid (HNO3) are added, and all is mixed during 3 hours. Finally, the CS2 is decanted and passed through a silica gel column. It is worth noting that, after using the solvent, a chromatographic test is made to assure the elimination of benzene traces.
Selection of the Internal Standard (IS)
With the purpose of correcting the effects and the loss of the analyte while preparing the sample, it was decided to employ an internal standard intended to improve BTEX quantification. According to [14], a general recommendation for the election of the internal standard is based on the chemical similarities of the compounds involved. Among others, the following compounds were taken into consideration for defining the internal standard: cyclohexane, tert-butyl alcohol, diethylene glycol, Dinonylphthalat, diethylene glycol ethyl ether, and fluorobenzene.
Chromatographic conditions
After a bibliographic review and after performing relevant laboratory tests, optimum chromatographic conditions to achieve selectivity were the following: Detector and injector temperature at 250 oC; furnace temperature ramp: initial (32 oC / minute), from 1 oC / minute to 45 oC, then 8 oC / minute up to 65 oC / minute, then 10 oC / minute up to 210 oC; carrier gas: Ultra-pure helium, 99.99%; flow: 1.37 mL/min; mode of injection: split (5:1); and injection volume: 1 µL. An HP-INNOWAX Chromatographic Column was used; 30 m x 0.250 mm ID, 0.25 um and Flame Ionization Detector.
Preparation of standard solutions
From a BTEX certified standard of 2,000 µg/mL, preparation of work standard solutions was made at known concentrations of carbon disulfide and the internal standard was added. The internal standard work solution (Flourobenzene) is prepared in acetone from its certified standard of reference. Then, each BTEX standard and the sample extract are added with the same amount, in such a way that it keeps a constant concentration in all solutions. Standards are stored in amber glass containers and kept under refrigeration.
Extraction and chromatographic analysis
The extraction and analysis process is performed as follows: The adsorbing material (activated charcoal sample) is taken to a 4.0 mL vial and is then added with 3.0 mL of purified CS2 and a volumetric amount of fluorobenzene standard (internal standard) of known concentration, which is in function of the expected BTEX concentration. Later, the vial is covered to avoid volatilization and stirred during a minute in vortex; then, it is subject to an ultrasonic bath for 30 minutes. Finally, it is centrifuged during 5 minutes at 3,500 rpm and injected with 1.0 µL of the extract supernatant in the GC/FID.
Determining BTEX concentration in the extract
For determining BTEX concentration, calibration curves are built for each analyte. The process is performed through the chromatographic analysis of a blank of reagents and a series of standards prepared from a BTEX certified standard of 2,000 µg/mL with internal standard (Fluorobenzene) in carbon disulfide solvent. Determination requires the construction of two calibration curve intervals; one interval within the low range of 0.3 and 12.0 µg/mL concentrations, and another one within the high range of 0.3 and 97.0 µg/mL concentration, with seven levels of concentration per each curve.
For the concentration estimation of compounds (µg/mL), the following steps should be taken: a known BTEX concentration standard is prepared, containing fluorobenzene as the internal standard, at the same concentration added in the sample. Then, it is injected in the chromatograph in triplicate, and its calculation is made based on the equations below: Calculation of the Response Factor (RF) for each analyte of the BTEX standard (see Eq. (1))
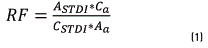
Where RF is the response factor of the analyte of interest; ASTDI is the area of the internal standard (Fluorobenzene) in the BTEX standard; CSTDI is the internal standard (Fluorobenzene) concentration in the BTEX standard; Aa is the area of the analyte of interest in the standard; and Ca is the concentration of the analyte of interest in the standard. Then, after obtaining the RF of the standard, the analyte concentration is calculated according to Eq. (2).

Where Cam is the concentration of the analyte in the sample; RF is the response factor of the analyte of interest; ASTDI is the area of the internal standard (Fluorobenzene) in the sample; CSTDIm is the internal standard (Fluorobenzene) concentration in the sample; and Aam is the area of the analyte of interest in the sample.
2.4. Chromatographic method validation Method selectivity and specificity
Recognition of each compound is performed by injecting the standard that contains a mixture of analytes of interest, duly prepared from the BTEX certified standard of 2,000 µg/mL and the individual injections of each analyte (BTEX). During the specificity tests, a blank extracted from the sample (activated charcoal) is injected with the purpose of checking that there is no interference or that other compound does not co-elute with the analytes of interest.
Method detection limit and quantification
The Method Detection Limit (MDL) has been experimentally set by employing a standard of 0.300 µg/mL; for this purpose, seven (7) standard solutions of such concentration are prepared and each solution is injected in triplicate. With the information obtained and applying Eq. (3) below, results of the MDL are estimated:
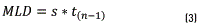
Where MLD is the method detection limit; s is the standard deviation; t(n-1) is the t-Student distribution for n-1 with a confidence of 95% t-Student (n=7): 1.943. In order to estimate the Method Quantification Limit (MQL), Eq. (4) below is applied. In this sense, the MQL is established as a value ranging between 1 and 10 times the method detection limit. In this case, the MQL is established at 6 times the MD, as follows:
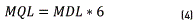
Accuracy
Accuracy of the method is estimated through repeatability of 10 replicas of BTEX solutions. That is, it is performed at two levels of concentration: 1.0 and 50.0 µg/mL. The acceptance criterion for accuracy is that the Relative Standard Desviation (RSD) should be lower than 10% [15, 16].
Exactness
In relation to the analytical methods, exactness is known as the match between the average of a set of results or of an individual results and the value accepted as true or correct for the amount measured [15]. It is performed at two levels of concentration: 1.0 µg/mL and 50.0 µg/mL. It is established by estimating the percentage of error according to Eq. (5) below:
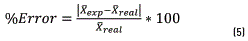
In the equation above, values of 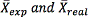 , are in turn: The average of experimental results and the value accepted as true; the acceptance criterion for exactness is that the percentage of error should be lower than 10%.
, are in turn: The average of experimental results and the value accepted as true; the acceptance criterion for exactness is that the percentage of error should be lower than 10%.
Linearity
Linearity is defined as the capacity of the analytical method to produce results directly proportional to the concentration or amount of analyte within a defined range [15]; in this sense, linearity determination of the method requires the preparation of solutions of the analyte mixture at two intervals of concentration; a low one, between 0.3 µg/mL and 12.0 µg/mL, with six levels of concentration, and a high one, ranging between 0.3 µg/mL and 97.0 µg/mL, with five levels of concentration. Five solutions are prepared for each level; each level having the same concentration of the internal standard, and then such solutions are injected in triplicate in the gas chromatograph.
Recovery percentage
The assessment of recovery is performed from activated charcoal samples enriched with the analytes of interest and three different concentrations: low level of 5.0 µg/mL, medium level of 12.0 µg/mL, and high level of 50,0 µg/mL. Concentrations obtained from these samples are compared to the directly prepared standards, at the same levels of concentration. Eq. (6) is applicable to the recovery percentage.

Robustness
For this research, Robustness has been assessed for benzene, toluene, and m-xylene through the laboratory-laboratory comparison.
2.5. Selection of activated charcoal and construction of a passive sensor
In the selection and construction of passive sensor, four different types of coal were evaluated as follows: activated coal for a CO2, manufactured from bituminous mineral coal; averaged MACRO activated charcoal; averaged activated charcoal 50/60, and granular activated charcoal CGC produced from coconut shell and activated with water vapor. The origin of studied charcoals corresponds to national samples, subject to BTEX compounds adsorption and recovering processes in the laboratory. Each coal was subject to the following BTEX vapor mass: 1.5 mg, 4.5 mg and 15.0 mg, during one day of exposition. Later, through an extraction validated methodology and adsorbed BTEX chromatographic analysis, the quantity of compounds present is investigated for each studied coal, the recovering percentage was evaluated and the one with the highest value was selected.
Finally, the preparation of passive samplers is initiated (it consists in a glass cylinder – internal diameter 8.1 mm x a length of 22.0 mm- open at the extremes, inside which a specific quantity of selected material is placed, which ranges from 0.12 to 0.15 grams of charcoal. Spreading distance was limited from a layer of cellulose acetate in order to maintain the charcoal inside the cylinder.
Methodology of sampling in field
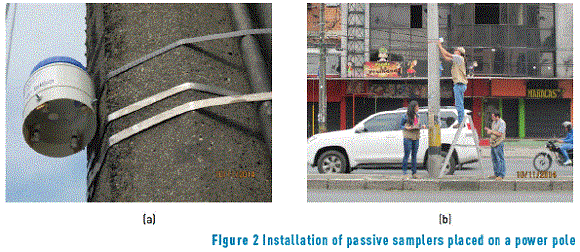
In order to avoid contamination, and deterioration in the samples (rain, bird droppings, etc.), of passive samplers located for BTEX evaluation in heave traffic roads, a closed system was used in each point, coupled to a housing prepared for the conservation of samples. Housings were built in polyethylene and have a lid in the higher part and some internal clamps, where such passive samplers are located (see Figure 2(a)).
Installation of passive sampling samplers
Polyethylene housings with corresponding passive samplers were installed at an average height ranging from 2.5 to 3.0 meters (see Figure 2b), in 21 points along a heavy traffic road (six lines) with an approximated distance of 3 km. In each point on the road, two passive samplers per site were placed and six were placed per transversal section (east, central, and west sidewalk), for a total of 42 samplings. Measurement time was adjusted to recommendations provided in prior studies [1, 12].
Sample conservation
Passive samplers installed during the research complied with a manipulation strict control (beginning and ending); that is to say, at the beginning they were closed until they were assembled in the sampling site; at the end, they were disassembled, closed, and refrigerated at a temperature lower than 5 °C, in order to avoid volatilization of captured compounds.
Determining BTEX concentration outside
After passive sample takers were exposed on the way for 28 days, adsorbent material BTEX was extract to be analyzed, in order to determine the total mass of each one of the analytes trapped in the getter (see Eq. 7).
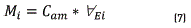
In which Mi is the total mass of the analyte i, in µg; Cam is the concentration of analyte i in the liquid extract, in µg/mL; 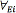 is the volume of liquid extract in µL. The average concentration of the analyte in the air matrix for the sampling period, is determined as follows [17]; (see Eq. 8):
is the volume of liquid extract in µL. The average concentration of the analyte in the air matrix for the sampling period, is determined as follows [17]; (see Eq. 8):

In which, Xi is the average concentration in the air matrix of the analyte i, in µg/m3; Mi is the total mass of the analyte i, in µg; Q is the capture rate of the analyte in the air matrix, in m3/d; and t is the period of time – in days- that the passive getter has been exposed in the sampling place.
3. Results and analysis
3.1. Selected extraction solvent
Among the extraction solvents assessed, the carbon disulfide was selected since it shows a high desorption efficacy for aromatic hydrocarbons adsorbed in activated charcoal, exceeding that of acetone and methanol. Additionally, carbon disulfide is characterized for its stability when stored at 5 oC and for a period of time not exceeding 30 days; besides, it shows good solubilization properties for many analytes and a very low response in the FID [18, 19].
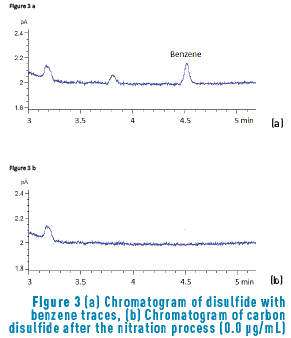
In tests performed during this research, it was found that carbon disulfide contained benzene traces. Such information can be verified with Figure 3(a). This figure is enlarged in order to show found traces. In this sense, presence of the compound is an obstacle for determining the compound; for this reason, a chemical nitration process was implemented, elimination of solvent benzene was achieved. Later, benzene removal was evaluated (see Figure 3(b)). When carbon disulfide is chromatographically analyzed, a chromatogram free of benzene traces can be observed.
3.2. Selection of Internal Standard (IS)
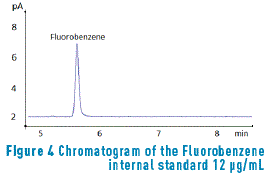
During the selection study of the internal standard, different compounds, which selection is described below, were evaluated: Cyclohexane and Tert-Butyl Alcohol. Both compounds showed interference with the benzene retention time. When Diethylene Glycol and Dinonylphthalat were employed, the presence of traces that could interfere with compounds of interest was observed and the diethylene glycol ethyl ether was studied; despite a good chromatographic response was observed, it was determined that it is a compound adsorbed by the activated charcoal that is the same matrix to be evaluated; hence, it is not reliable to be used in its quantification. Finally, the fluorobenzene was tested, as shown in Figure 4, and it was possible to find better results and a well differentiable signal of all other compounds; therefore, the fluorobenzene was chosen as the internal standard for quantifying BTEX concentrations in the activated charcoal samples.
3.3. Response factors
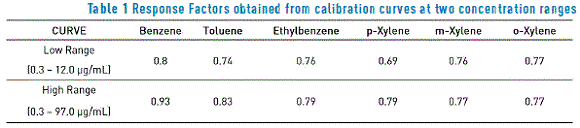
During the validation period, response factors were calculated for two validated calibration curves, with the purpose of assuring the method linearity. As indicated above, one curve was located within a low range (0.3 – 12.0 µg/mL concentration) and the second curve was located within a high range (0.3 – 97.0 µg/mL concentration), responses of which are described in Table 1 below. These factors are important because they are part of the constants used for quantifying BTEX compounds.
3.4. Validation
Method selectivity and specificity
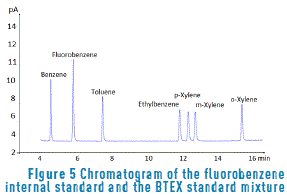
According to the method, and just as described in Table 2, it was observed that BTEX showed clearly differentiable retention times under the chromatographic conditions described above.
Figure 5 shows the chromatogram of compounds; the internal standard and response times of each analyte can be clearly distinguished. On the other hand, when a chromatogram of the sample extracted blank was made, it was found that there were no traces that could interfere with the analytes of interest. From these results, it was determined that this is a selective and specific method for analyzed compounds; that is, no interference associated to activated charcoal was seen and compounds are appropriately resolved and distinguished working under the conditions described above. The concentration of the standard mixture and internal standard was 6.7 mg/L.
Method detection and quantification limits
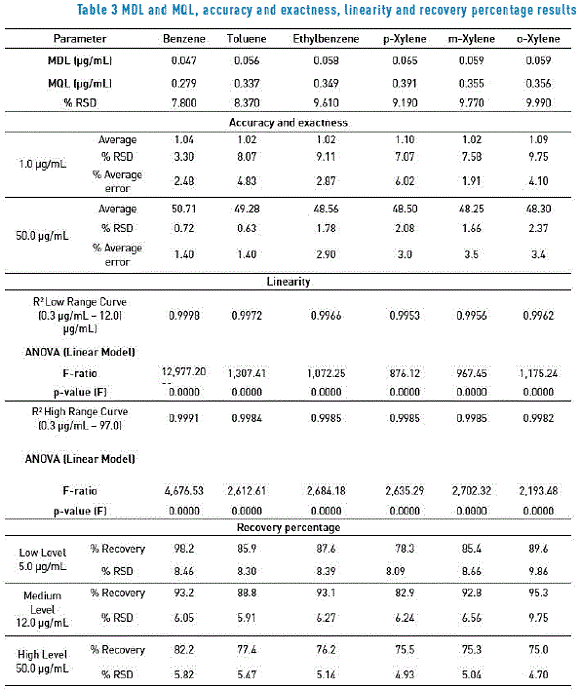
The method detection and quantification limits are shown in Table 3; such limits were obtained from the equations shown in 2.4 above. These limits are accepted as long as the % of Coefficient of Variations is lower than 10%. This criterion is fulfilled in all compounds.
Accuracy and exactness
Results obtained for accuracy and exactness are shown in Table 3. Such parameters, as indicated above, were assessed at two levels of concentration (1.0 µg/mL and 50.0 µg/mL). Therefore, the acceptance criterion for accuracy is that the coefficient of variation is lower than 10%; the acceptance criterion for exactness lies on the fact that the percentage of error is lower than 10%. Then, according to the results shown in Table 3 it can be demonstrated that this is an accurate and exact method for both levels of concentration.
Linearity
With the purpose of establishing the linearity method, construction of two calibration curves was proposed in this research for each solution, injected in triplicate in the gas chromatograph. With the data obtained, the following step was to find a calibration curve for each analyte, as follows: ratio of concentrations (analyte concentration / internal standard concentration) was taken to a graph versus the ratio of areas obtained (Analyte Area / Internal Standard Area), and the equation of the straight line for each analyte and its correlation values (or R2) was determined. As an example of the estimation, Table 4 shows the benzene linearity values for the low range. The acceptance criterion of R2 allows determining that when values ≥ 0.995 are obtained from low and high range curves, linearity of analyzed ranges can be accepted and since the P-value in the ANOVA is less than 0.001, there is a statistically significant relationship between X and Y, at the 99.9% of confidence level. In this sense, and according to description in Table 3, it can be affirmed that the assay is in compliance with the linearity patterns.
Recovery percentage
Table 3 shows the recovery percentages obtained for each evaluated analyte in the three levels of study. Table 3 shows that recovery percentages range between 70% and 110% (for the reference charcoal) and their coefficients of variation are below 20%; then, it can be concluded that this is an efficient method to extract the matrix analytes.
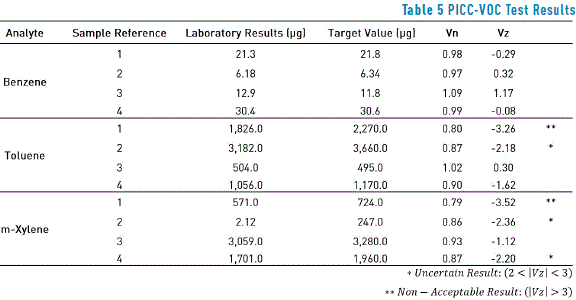
Robustness
The method robustness was evaluated through international inter-calibration tests (PICC-VOC Tests; Quality Control Inter-Laboratory Program; Ministry of Employment and Social Security. Spain Government). Consequently, concentration values in passive samplers of benzene, toluene, and m-xylene are expressed in Table 5. Uncertain or non-acceptable results can be explained for the loss of analyte at any time during the process, due to its volatile nature; please note that these results are lower than the target value in all cases.
For compounds such as ethylbenzene, o-xylene, and p-xylene no test has been made since the inter-calibration program does not offer them among the compounds to be evaluated.
3.5. Activated charcoal selection
Once the charcoal samples were analyzed, coconut – origin charcoal was selected, due to its better recovery of BTEX (89.1), in percentage. Table 6 shows corresponding statistical answers. In Table 7, recovering percentages of each BTEX compound are shows in CGC coal.
3.6. BTEX concentration in a heavy traffic road
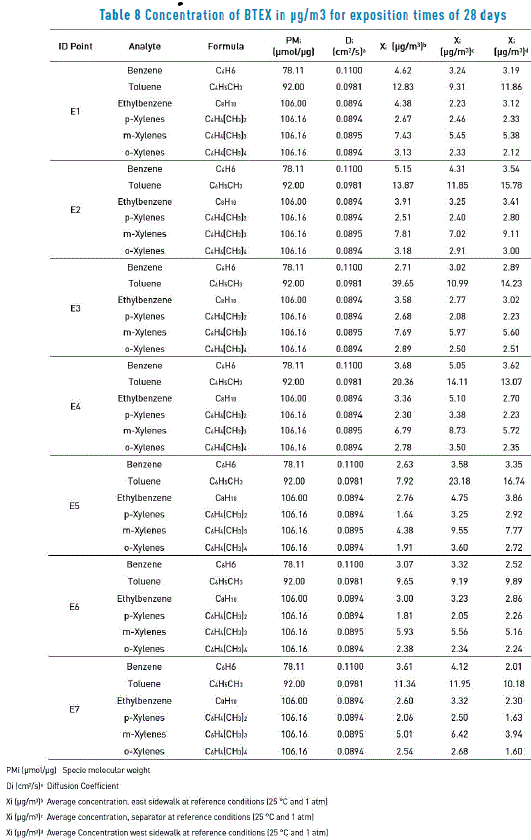
Table 8 shows results obtained at the end of laboratory determinations. Each monitoring point registers answering values taken by duplicate (two passive samplers per site) and each extraction in laboratory was analyzed by triplicate. Resulting data correspond to a sample on the road (east sidewalk, center and west sidewalk), after the statistical management of obtained results. In Table 8, it is observed that the compound with more answers in terms of concentrations is toluene, followed by m-Xylenes
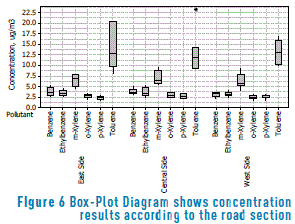
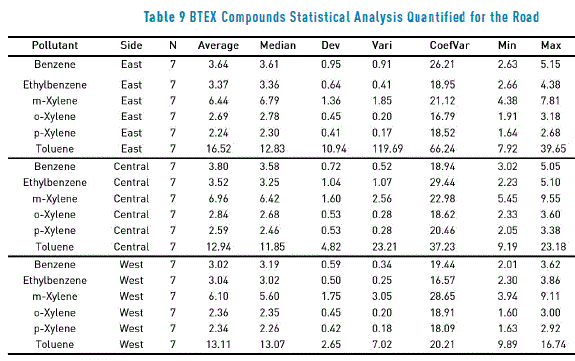
On the other hand, in Figure 6, results of concentrations per contaminant (analyte), obtained from a sample collected on calle 33 (Medellín, Antioquia, Colombia) for each one of the passive samplers (East, Center and West,) are presented in a Box-Plot diagram. The figure shows a bigger dispersion of results for the toluene, a compound which, at the same time, registers the biggest values in concentration. In Table 9, statistical analysis results performed to air quality data, obtained on a heavy traffic road are presented.
Figure 6 and Table 9 show a low variation between average values and median, which means a good response of CGC charcoal for the contaminants response at the studied zones4. Conclusions
- According to the results shown in this article, carbon disulfide (CS2) can be defined as an optimum solvent for extracting BTEX adsorbed in activated charcoal. Due to the benzene traces present in the carbon disulfide, it requires a preliminary cleaning known as benzene nitration and a future verification before using it.
- The fluorobenzene standard is in compliance with the fundamental conditions of an internal standard to be used for the quantification of BTEX.
- This research was useful to validate the analytical method for quantifying BTEX adsorbed in activated charcoal using the internal standard. In this sense, it was found that this is a selective, specific, linear, accurate, exact method with recovery percentages ranging between 75.0% and 98.2% for all analytes. Therefore, the method complies with the acceptance criteria and the results obtained with the application of the method will be highly reliable.
- From the inter-laboratory exercise performed by participating in PICC tests, it was possible to show that this is a robust method for benzene, toluene, and m-xylene.
- This research allows determining in an easy and precise way a high reliability level, the concentration of volatile organic compounds (BTEX type) in samples taken in the environmental.
- A laboratory technique, which will allow developing countries to detect the presence of high environmental impact species with enough precision at low costs, such as the organic compounds (BTEX), was established.
- CGC charcoal used as an adsorbent means registered data which adjust to experimental conditions analyzed at laboratory level. In this sense, the biggest concentration values correspond to Toluene, followed by m-Xylene.
5. References
1. A. Buczynska et al., ''Atmospheric BTEX-concentrations in an area with intensive street traffic'', Atmospheric Environment, vol. 43, pp. 311-318, 2009. [ Links ]
2. A. Hinwood et al., ''Risk factors for increased BTEX exposure in four Australian cities'', Chemosphere, vol. 66, no. 3, pp. 533-541, 2007. [ Links ]
3. M. Lacasaña, B. González, M. Rodríguez and D. Daponte, ''Evaluación de la exposición de BTEX en el campo de Gibraltar'', Escuela Andaluza de Salud Pública, Granada, Spain, Report, Jun. 2008. [ Links ]
4. G. Demirel, O. Özden, T. Dögeroglu and E. Gaga, ''Personal exposure of primary school children to BTEX, NO2 and ozone in Eskişehir, Turkey: Relationship with indoor/outdoor concentrations and risk assessment'', Science of the Total Environment, vol. 473-474, pp. 537-548, 2014. [ Links ]
5. S. Lee, M. Chiu, K. Ho, S. Zou and X. Wang, ''Volatile organic compounds (VOCs) in urban atmosphere of Hong Kong'', Chemosphere, vol. 48, no. 3, pp. 375-382, 2002. [ Links ]
6. Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological Profile for Benzene. Atlanta, USA: U.S. Department of Health and Human Services, Public Health Service, 2007. [ Links ]
7. J. Acevedo, '''', M.S. thesis, Universidad San Francisco de Quito, Quito, Ecuador, 2006. [ Links ]
8. M. Fernandes, L. Brickus, J. Moreira, and J. Cardoso, ''Atmospheric BTX and polyaromatic hydrocarbons in Rio de Janeiro, Brazil'', Chemosphere, vol. 47, no. 4, pp. 417-425, 2002. [ Links ]
9. Y. Kerchich and R. Kerbachi, ''Measurement of BTEX (Benzene, Toluene, Ethybenzene, and Xylene) Levels at Urban and Semirural Areas of Algiers City Using Passive Air Samplers'', J. Air Waste Manage. Assoc., vol. 62, no. 12, pp. 1370-1379, 2012. [ Links ]
10. H. Pfeffer, ''Ambient air concentrations of pollutants at traffic-related sites in urban areas of North Rhine-Westphalia, Germany'', Science of the Total Environment, vol. 146-147, pp. 263- 273, 1994. [ Links ]
11. C. Sánchez, R. Quijano, E. Molina, C. Rubiano and G. Londoño, ''Fortalecimiento de la red de monitoreo de calidad del aire en el Valle de Aburrá con medidores pasivos'', Revista Gestión y Ambiente, vol. 11, no. 1, pp. 67-84, 2008. [ Links ]
12. K. Elke, E. Jermann, J. Begerow and L. Dunemann, ''Determination of benzene, toluene, ethylbenzene and xylenes in indoor air at environmental levels using diffusive samplers in combination with headspace solid-phase microextraction and high-resolution gas chromatography-flame ionization detection'', Journal Chromatography A, vol. 826, no. 2, pp. 191-200, 1998. [ Links ]
13. Occupational Safety & Health Administration (OSHA), Benzene, OSHA Method ORG-12, 1980. [Online]. Available: https://www.osha.gov/dts/sltc/methods/organic/org012/org012.html. Accessed on: Jun. 27 2013. [ Links ]
14. I. Zenkevich and E. Makarov, ''Chromatographic quantitation at losses of analyte during sample preparation: Application of the modified method of double internal standard'', Journal of Chromatography A, vol. 1150, no. 1-2, pp. 117-123, 2007. [ Links ]
15. L. Huber, Validation of Analytical Methods. Germany: Agilent Technologies, 2010. [ Links ]
16. American Public Health Association (APHA), American Water Works Association (AWWA) and Water Environment Federation (WEF), Standard Methods for the Examination of Water and Wastewater, 22nd ed. Washington, D.C., USA: APHA, 2012. [ Links ]
17. United States Environmental Protection Agency (EPA), ''Passive Samplers for Investigations of Air Quality: Method Description, Implementation, and Comparison to Alternative Sampling Methods'', U.S. Environmental Protection Agency (EPA), Washington, D.C., USA, Engineering Issue Paper, EPA 600-R-14-434, Dec. 2014. [ Links ]
18. P. Eller, NIOSH manual of analytical methods, 4th ed. Cincinnati, USA: National Institute for Occupational Safety and Health (NIOSH), 2003. [ Links ]
19. M. Ras, F. Borrull and R. Marcé, ''Sampling and preconcentration techniques for determination of volatile organic compounds in air samples'', TrAC Trends in Analytical Chemistry, vol. 28, no. 3, pp. 347-361, 2009. [ Links ]