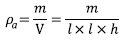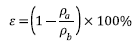1. Introduction
Electrospinning is an old technique. It was first observed in 1897 by Rayleigh, studied in detail by Zeleny on electrospraying in 1914, and patented by Formhals in 1934 [1]. The electrospinning process may be considered the most promising of all nanotechnologies, due to versatility and cost to produce nanofibers [2, 3], with large surface area, porosity, orientation, and dimensions in a controlled manner with excellent mechanical and easy functionalization properties for multiple applications [2] [3-8]. This technique has a variety of applications such as it is shown in Figure 1.
Due to the biodegradable nature and exceptional mechanical properties of polycaprolactone (PCL), this has been used in multiple applications [9]; some of these are shown in Figure 2. In the last two decades, PCL and other bioabsorbable polymers have found their main application for the biomedical area, such as devices that provide structural support in tissue engineering, controlled drug release constituent materials in various nanocomposites [9-12].
The production of nanofiber has recently garnered much attention due to their potential applications [1,10]. These nanofibrous polymers are unique due in part to their superior mechanical properties, large surface area to volume ratio, and their potential to resemble cellular topographies [10]. Nonwoven polymer fibers in the nanometer scale are being investigated currently for uses of composites reinforcements, filtration, chemical sensors, drug delivery and tissue scaffolding [1, 10]. The diameter and morphology of the nanofibers have an important role in the performance of these materials [13].
Polycaprolactone fibers have been developed by different authors for multiple applications, we will see some of them. Karuppuswamy and Reddy [14] fabricated polycaprolactone nanofibers with different concentrations of the antibiotic drug (tetracycline hydrochloride 2%, 3%, 4% and 5%) for the controlled drug delivery. The smaller diameter fibers obtained by these authors were of 419 nm without drug and 643 nm with 2 % of tetracycline hydrochloride [14]. Madhaiyan et al [15], produce a biocompatible PCL (Mw=80,000 g·mol-1) polymer nanofiber mediated sustained release of the hydrophilic drug (Vitamin B12) and applicability as the transdermal delivery system is attempted. The fiber diameter was within the range from 700 nm to 2,500 nm. McEachin and Lozano [10] use the forcespinning technology to produce polycaprolactone nanofibers (Mw=60,000 g·mol-1) with an average diameter of 220 nm with a standard deviation of 698 nm. Lopez et al. [16], produce polycaprolactone fibers (Mw=80,000 g·mol-1) with silver nanoparticles and antimicrobial activity against Gram-Positive and Gram-Negative Bacteria, the minimum diameter of the fibers obtain for these authors was 159±79 nm with 100 mM of silver nanoparticles. Chen et al. [17], prepare composite nanofibers of polycaprolactone (Mw=80,000 g·mol-1) and nanohydroxyapatite for osteogenic differentiation of mesenchymal stem cells with similar diameters 340±30 nm. Sultanova et al. [18], produce polycaprolactone (Mw=80,000 g·mol-1) fibers to control the release of the hydrophilic drug (ampicillin) using a coaxially electrospun, the nanofibers in this study had average diameters of 702±166 nm, 464±214 nm, and 567±180 nm.
Materials made with biodegradable polymers depend directly on the characteristics of shape, size, and composition of their own [19]. As shown by multiple studies, polycaprolactone fibers have been used by different applications, although in many papers they describe nanofibres, in none they report fibers with the average diameter under 100 nm. Because this reason, the aim of this study was to determine the influence of molecular weight of polycaprolactone, solvents and operational conditions to obtain the most quantity of nanofibers under 100 nm of diameter by the electrospinning process.
2. Materials and methods
2.1. Materials
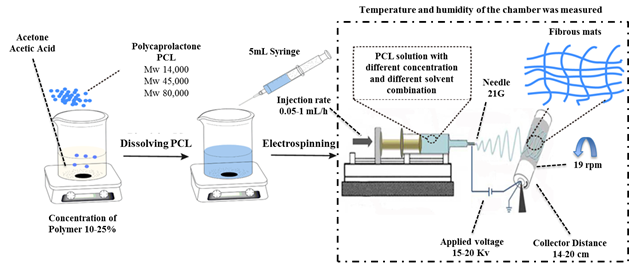
PCL with three different molecular weights (Mw) 14,000 g(mol-1, 45,000 g(mol-1 and 80,000 g(mol-1 was used, all polymers were purchased from Sigma-Aldrich. The acetone and acetic acid were purchased from Merck.
2.2. Electrospinning
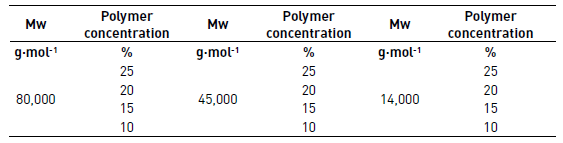
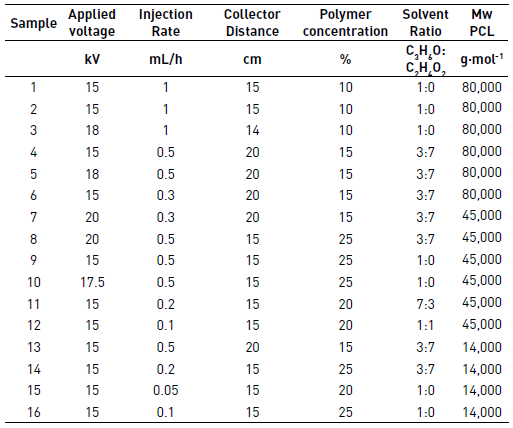
PCL solutions with three different molecular weights (Mw) 14,000 g(mol-1, 45,000 g(mol-1 and 80,000 g(mol-1 were prepared at concentrations of 10%, 15%, 20% and 25% of the polymer in acetone to be evaluated qualitatively in the process of electrospinning and defined if these solutions had the potential to be electrospun. Each solution was taken to the electrospinning equipment to 15 kV, an injection rate of 0.5 mL/h and the collector was positioned to 15 cm. The solutions for qualitative evaluation of electrospinning are shown in Table 1.
In this study, the polymers were dissolved in different mixtures of acetone and acetic acid at a concentration of 10% to 25% of the polymer before the electrospinning process. The experimental set-up is shown in Figure 3 and consisted in three syringes of 5 mL and three stainless steel needles 21G. The voltage used was of 15 to 20kV and the collector distance used was of 14 to 20 cm. The collector was covered with aluminum foil and the rotational speed of the collector was set up at 19 RPM.
Afterward that the electrospinning process is finished, the samples were air dried for at least 48 hours before characterization. The electrospinning experiments were performed at room humidity and room temperature. Table 2 shows the test conditions for each experiment.
2.3. SEM
The study of the morphology of the fibers was performed using a Field Emission Electron Microscope (JEOL, JSM-7100F) with an accelerating voltage of 15 kV. The nanofiber samples were sputter coated with a 2 nm thick gold film before measurements. The fiber diameters were determined using Image J software. At least 250 nanofibers in different SEM images were analyzed for each sample.
2.4. Porosity of mats
The thickness of the mats was measured with a micrometer (INSIZE, IP54). The apparent density 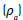 and porosity
and porosity 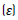 were determined using the Eqs (1) and (2) [19].
were determined using the Eqs (1) and (2) [19].
Where m and V are mass and volume of the mat respectively. The dimensions of the sample taken for the porosity mats are given by a square area (l X l) with thickness (h) and the bulk density of the polymer was 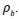 .
.
2.5. Mechanical properties
The mechanical properties of the mats of PCL were determined with a texture analyzer (TA TX-Plus) at room temperature; the speed of grip separation was 400 mm/min. All samples were prepared according to standard ASTM D882-12, due to equipment limitations, the specimen dimensions were 0.8 x 5 cm. The samples were analyzed in one direction, due to the dispersion of the fibers in multiple directions (unaligned), which allows considering the isotropic material. The thickness was measured with a micrometer (INSIZE, IP54), which could be determined 0.2 mm uniforms throughout the length of the sample.
3. Results and Discussion
3.1. Qualitative evaluation of electrospinning
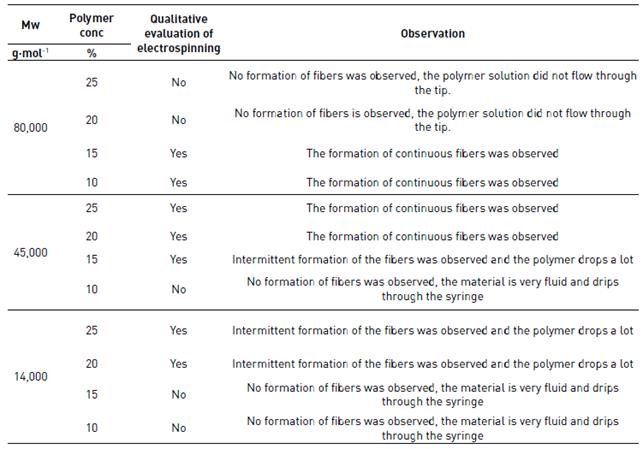
The results of qualitative evaluation of electrospinning solutions are shown in Table 3.
As shown in Table 3, some of the evaluated concentrations of the polymers were discarded for analysis because no fiber formation was observed, due to the high concentration (>20%) of high molecular weight polymer (80,000 g(mol-1) that does not allow the solution to flow through the tip or the low concentration (<15%) of medium (45,000 g(mol-1) and low (14,000 g(mol-1) molecular weight polymers with which are obtained very fluid solutions, that was not suitable for electrospun.
3.2. Morphology and size of the fibers
According to the literature, the morphology and diameter of fibers in the electrospinning process are dependent on the intrinsic properties of the polymer solution such as the type of polymer, the conformation of polymer chain or molecular weight, viscosity or concentration, elasticity, electrical conductivity, and the polarity and surface tension of the solvent [1, 2, 19]. Process parameters as the applied voltage, the injection rate, and the collector distance can also affect the properties of the fibers [2].
The molecular weight of the polymer has a significant effect on rheological and electrical properties such as viscosity, surface tension, conductivity and dielectric strength [1]. Due this, in this study, three different molecular weights of the PCL were evaluated.
First, the results of PCL of Mw 80,000 g·mol-1 under different operating conditions, second the result of PCL of Mw 45,000 g·mol-1 and finally the results of PCL of Mw 14,000 g·mol-1 were shown. The applied voltage during the electrospinning process has a strong effect on the stability of the fiber jet [1]. This can be observed in the first three tests with PCL of Mw 80,000 g·mol-1 in Figure 4. When the voltage approach 15 kV, it was found that polymer dropped down continuously from the tip of the needle. However, when the voltage was increased to 18 kV, the jet started to become stable, resulting in fiber without beads. The higher voltage can break the surface tension of polymer solution in a constant mode. However, when the voltage gets too high or too low, the ejection of the polymer would become unstable again [1, 19]. In the first case, due to the greater coulomb forces in the jet as well as a stronger electric field that breaks the stability of the jet, and in the second case, due to the lack of this field that does not allow the fibers to arrive at the collector [1].
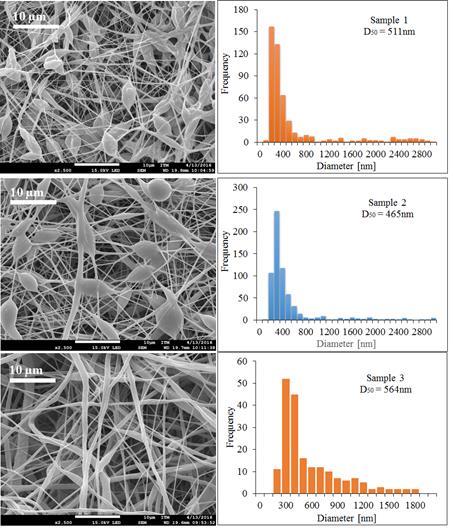
Figure 4 SEM micrographs and the diameter distribution histograms of the PCL Mw 80,000 g·mol-1 using acetone as solvent under different conditions, Samples 1, 2 and 3
Figure 4 exhibits the reduction of the beds in the electrospinning mats and the increment of the diameter of the fibers when increasing the voltage and decreasing the collector distance. The temperature and humidity did not have significant effects on the mats when working with acetone as the solvent because this evaporates easily.
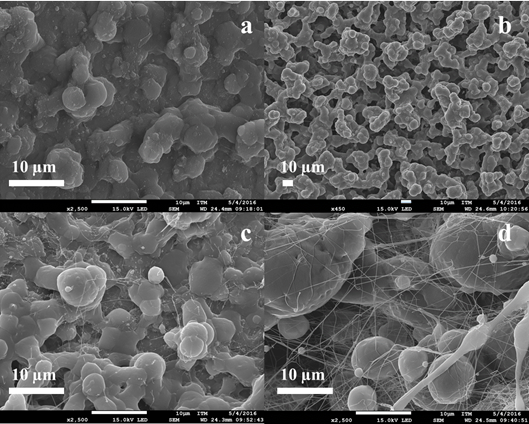
Solvents have an important role in determining the fiber size and morphology during spinning of polymer fibers because these affect directly the polymer solution [1]. Figure 5 exhibits the different behaviors of PCL of Mw 80,000 g·mol-1 under different operational conditions using a mixture of acetone and acetic acid as solvent.
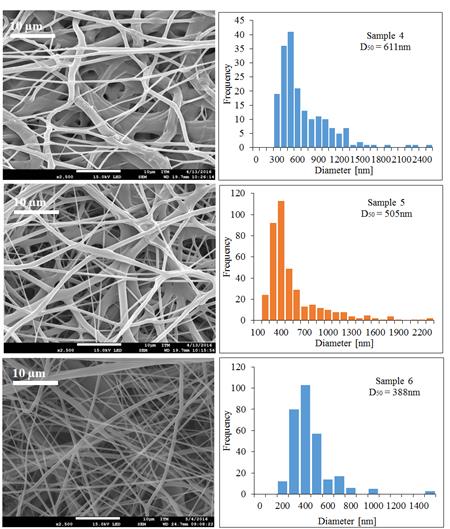
Figure 5 SEM micrographs and the diameter distribution histograms of the PCL Mw 80,000 g·mol-1 using acetone and the acetic acid mixture as solvent under different conditions, Samples 4, 5 and 6
In Figure 5, it is possible to see that when the injection rate decreases, the diameter of the fibers decreased and it is also possible to observe the appearance of the fibers changes when it injects much polymer solution, this is not enough to dry due to the presence of acetic acid in the polymer solution.
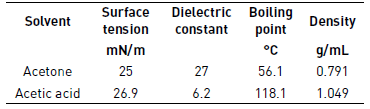
In Table 4, we have shown different properties of solvents such as surface tension, dielectric constant and boiling point that should be kept in mind in the electrospinning process.
The acetic acid has a higher boiling point than acetone as shown in Table 3, retarding the drying time of the fibers and affecting the morphology of these. In samples 4 and 5, it is possible to see more melted fibers than in sample 6, this is because of the injection rate, that is slower in sample 6, allowing the fibers to dry and do not melt together.
PLC trials with Mw 45,000 g·mol-1 were also performed, to see the effect of molecular weight and other operating conditions in the production of fibers. Sample 7 was not possible to electrospinning because the polymer concentration was very low, this caused that the polymer solution was very fluid and droplets dropped down continuously from the tip of the needle. The SEM images and diameter distribution histograms of samples 8 to 12 are shown in Figure 6.
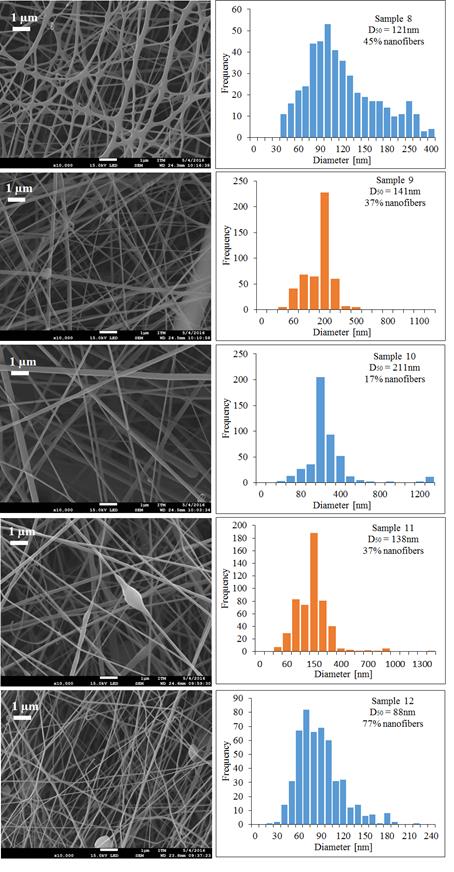
Figure 6 SEM micrographs and the diameter distribution histograms of the PCL Mw 45,000 g·mol-1 using different mixtures of acetone and acetic acid as solvent under different conditions
The variable that has more effect on morphology and diameter of the fibers of PCL of Mw 45,000 g·mol-1 was the injection rate, when this was low the electrospinning produced more nanofibers. This was done with the lower injection rate and yields fibers with a smaller diameter and more percentage of nanofiber (77%) in the sample. Increasing the applied voltage under the same operational conditions in the PCL of Mw 45,000 g·mol-1, samples 9 and 10 have the effect of increasing the fiber diameter, a similar behavior was reported by Duque et al. [20].
Despite the evaluation of different operating conditions such as polymer concentration, the rate of injection, collector distance and solvents used for PCL molecular weight of 14,000 g·mol-1, It was not possible to obtain fibers of this polymer solution, as it is possible to see in Figure 7. Sample 16, it is possible to observe small fibers with many clots.
PCL of Mw 14,000 g·mol-1 could be used in the process of electrospray to obtain nanoparticles to different applications [21].
3.3. Porosity of mats
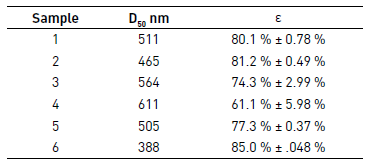
Porosity is an important parameter for nanofiber materials used for tissue engineering, filtration, protective clothing, drug delivery among others researchers become more interested in studying the unique properties of nanoscale materials [1, 19, 21-24]. Apparent density and porosity of electrospun PCL mats were calculated using Eq. (1) and (2), and are summarized in Table 5. In the case of PCL films of Mw 45,000 g·mol-1 and 14,000 g·mol-1 was not possible to determine the porosity, product of difficulty of separating the film of the aluminum foil due to its low thickness.
As reported in the literature, when the fiber diameter increases, the porosity of electrospun mat generally decreases, due to numerous fiber junctions caused by higher flow rate, as it is possible to see in Table 5 [1, 19].
3.4. Mechanical properties
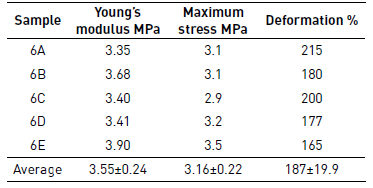
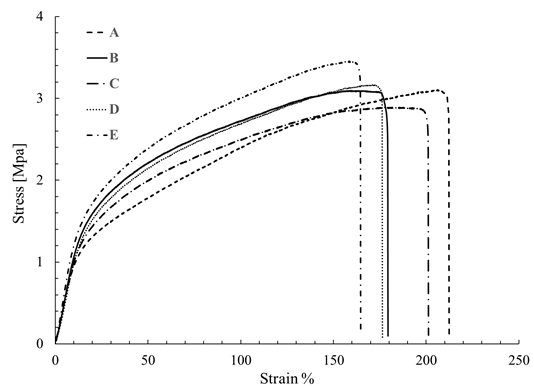
Sample 6 was selected for mechanical testing because this presented the best morphology regarding the other samples of PCL of Mw 80,000 g·mol-1 and lower average diameter of the fibers. Different fragments of Sample 6 were mounted on the texturometer, and then it rolled in order to determine the elastic modulus, the stress at rupture and deformation. These properties were tested by analyzing five samples; the results are presented in Table 6 and Figure 8 shows the curves of stress-strain. In the case of PCL films of Mw 45,000 g·mol-1 and 14,000 g·mol-1 was not possible to determine the mechanical properties, because the difficulty to separate the film of the aluminum foil due to its low thickness.
The average value found of Young's modulus was 3.55 Mpa, and strain to failure was of 187.4%. These values are similar to the values reported in the literature for films of PCL produced by electrospinning, Croisier et al. [25], obtained Young's modulus between 2.9 Mpa and 5.2 Mpa while Karuppuswamy and Reddy [14] obtained a value for tensile strength between 1.76 Mpa and 3.94 Mpa, and deformation of 105% to 141% for the polycaprolactone of 80,000 g·mol-1, however, this paper reported a decrease in the diameter of the fibers respect to such reports. As mentioned above, SEM images randomness in the distribution of the fibers is attributed to using a low-speed of collector and the absence of binding points affecting the mechanical properties observed. Moreover, the decrease of the mechanical properties of the scaffolds by electrospinning produced is likely to be due to the porosity between fibers, as described in the previous section varies between 60-85%.
In relation, the mechanical properties obtained for PCL (Mw 80,000 g·mol-1) films, these can be used in biomedical applications as scaffolds for tissue engineering, because in this application membranes able to bear high loads and sustain deformation are required [14], as shown in Table 4 and Figure 8.
4. Conclusions
The polymer solution (solvents and concentration of the polymer) and processing parameters such as molecular weight, applied voltage, and collector distance have a significant effect on the fiber morphology and diameter. Therefore, by manipulation of these parameters, one can get desired properties for the specific application. Low injection rates promote that the fibers with a smaller diameter, additionally this condition also causes that the solvent evaporates completely because the fiber takes more time to reach the collector. Furthermore, the use of acetic acid as a solvent reduce the beads in the electrospinning mats. However, the presence of the acetic acid increases the time to dry of the fibers, making necessary to increase the distance of the collector to facilitate the evaporation of the solvent to prevent the fuse of the fibers. On the other hand, increasing the collector distance can cause the fibers to break before reaching the collector. Consequently, knowing how these variables affect the electrospinning process becomes relevant since a balance between all the parameters is required to guarantee the quality of the fibers. Moreover, the molecular weight of the polymer has a significant effect on diameter and morphology of the fibers. High molecular weight promotes the obtaining fibers with a larger diameter and when the molecular weight decreases the fibers decrease their size. This study found that to obtain microfibers, it is recommended PCL with high molecular weight (Mw 80,000 g·mol-1), whereas to obtain more percentage of nanofiber, it is recommended PCL with medium molecular weight (Mw 45,000 g·mol-1). It was also found that PCL with Low molecular weight (Mw 14,000 g·mol-1) is not recommended to produce micro and nanofibers using the electrospinning technique.
In terms of porosity, it was found that as fiber diameter increases the porosity of the fibers decreased. This is related to the superficial area of the mat that is higher when the average diameter of the fiber is smaller. Mechanical properties of the films become more relevant depending on the application, with the results found in this study, it can be said that these films have the potential to be used as scaffolds for tissue engineering and other applications where mechanical properties as high strengths and high deformation capacities are required.
Through careful handling of the electrospinning variables, it is possible to obtain micro and nanofibers of PCL with an excellent morphology that fit the specific needs of each application.
The researchers hope to continue working on electrospinning films of bioabsorbable polymers and perform degradation studies and how the properties of these materials change over time to implement developments based on these films.
5. Author contributions
Gabriel Colmenares and Lina Hoyos conceived and designed the experiments and supervised the work. Liliana Agudelo, Yeixon Quintero, and Luis Rodriguez produced the mats in the electrospinning process. Yeixon Quintero and Luis Rodriguez executed the mechanical measurements. Liliana Agudelo realized the porosity measurement. Gabriel Colmenares and Yeixon Quintero carried out the SEM analysis. Gabriel Colmenares performed analyzed the data and wrote the paper.