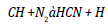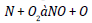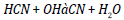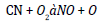1. Introduction
The global cogitations for biodiesel fuel use are blooming with crude oil prices extending high levels. Biodiesel is produced from animal fats and vegetable oils by discrete esterification processes. The production of biodiesel is supplemented by 219.5% from 7828.4 millionliters in 2006 to about 17179.09 millionliters in 2009 [1]. Palash et al. [2] scrutinized the impacts of biodiesel fuels on NOx emissions and their reduction commences. They found that biodiesel and its blends have ardent impacts on HC, CO, and PM emissions owing to the NOx influencing factors of this fuel. NOx formation can be delineated by three mechanisms, i.e., thermal NOx, fuel NOx, and prompt NOx. Thermal NOx is the predominant correspondent to NOx emissions from a diesel engine [3]. Thermal NO formation is influenced by higher combustion and peak flame temperatures throughout complete combustion. High combustion temperature which may be above 1800°C, shatters the hefty triple bond of nitrogen molecules and initiates thermal NOx [4]. Mostly, generation of NOx rely on physical and chemical properties of fuel [5].
McCormick et al. [6] explored various of tackles for reduction of NOx emissions from biodiesel. They detailed cetane improvers di-tert-butyl peroxide (DTBP) and Ethyl hexyl nitrate (EHN) are effectual for reducing NOx emissions (4%) in B20 blends. On the contrary, increasing fuel density, number of double bonds and quantified iodine number are equalized with increasing NOx emissions. They also accomplished that the properties of cetane number, density and iodine number are eminently correlated with one another. Gan and Ng [7] examined the eventual of antioxidants equallytert-butyl hydro quinone (TBHQ), butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) are commingled in assorted concentrations (250,500,750 and 1000 ppm) in B10 and B20 blends of fuel. They examined that BHA is a covenanting antioxidant for reducing CO and NO emissions contemporaneously in the combustion of biodiesel blends. TBHQ is equally adroit of terminating NO formation more at the disbursement of higher level of incomplete combustion.
Varatharajan et al. [8] explored the impact of antioxidant additives on NOx emission of jatropha biodiesel in diesel engine. The antioxidant additives α- tocopherol acetate, BHT, p-phenylenediamine, ethylenediamine and L-ascorbic acid are tested on single cylinder diesel engine. The experimental results exhibited that a 0.025%-m concentration of p-phenylenediamine additive is exquisite as NOx levels are decreased extremely comparable to sole jatropha biodiesel. While, the extension of antioxidants to neat biodiesel increased the CO and HC emissions. Conventionally, an antioxidant is a molecule which impedes the oxidation of other molecules. Free radicals are formed in oxidation and it is the main concern for the NOx generation in biodiesel combustion [9]. Antioxidants are computed to oxidizable organic materials to retard oxidation and to perpetuate the proficient entity of the substrates [10].
Occasionally, the biodiesel and concoction of antioxidant fuel conquers the peroxyl free radical dispositions by reaction with aromatic amines. These peroxyl free radical generations are the focal source for the greater biodiesel NOx emissions. A free radical is the unpaired electron of a molecule and that monitors the rate of reaction oxidation [11]. Furthermore, antioxidants can reduce free radical disposition by four routes: chain breaking reactions, chelating the transition metal catalysts, scavenging the inaugurating radicals and reducing the combination of reactive radicals [12]. The empirical of this study is to peruse the NOx fuelled in blends of mango seed biodiesel. Antioxidant additives are chosen hinged on the effectiveness, cost and availability using the amended concentration proposed from the subsisting work. Utterly, the antioxidant additives are acquired from Lab chemicals at Chennai, India.
2. Materials and methods
2.1. Test fuels
Mangifera induce is a comrade of the family of Anarcardiaceae and a sort of the genus Mangifera.It is customarily found in India, Pakistan and Philippines. All the antioxidants are weighed employing a high-precision electronic weighing balance and added to assessed quantity of mango seed biodiesel. To make 0.005%-m of antioxidant mixture, 50mg of antioxidant is appended to 1 kg of biodiesel. A 3000 rpm speed mixer is operated to devise a homogenous mixture of fuel and antioxidant. The antioxidants are mixed with B100 and B20 fuels at different concentrations such as 0.005%-m, 0.010%-m, 0.025%-m, 0.05%-m, 0.10%-m (%-m denotes molar concentration) with a constant engine speed of 1800 rpm. In this study, the reference fuel (B20) is produced by splash-blending method, which is the most perennial and cheapest process. The selected blending level is most incessantly used fraction in persistence and meticulous level biodiesel blend. Biodiesel is moderately compatible with diesel fuel and no phase separation is visually perceived in the blends [7]. The majority of the blending is splash blending each of two in the tanker of fuel or the tank of storage where the biodiesel and diesel fuel are pumped concomitantly into the tank [13].
2.2. Biodiesel production
Transesterification of mango seed oil is executed under emanating conditions: 3.82 gram potassium hydroxide (KOH) catalyst per liter of mango seed oil, 5:1 molar ratio of methanol to mango seed oil, 55°C internal reaction temperature and 2 hour reaction time. After 8 hours of transesterification process, the glycerine is detrained in the bottom layer, and the upper layer composed the pure biodiesel. The glycerine is detached from the mixture, and the pure biodiesel is sent to a washing tank. The methyl ester is washed with pure water, which is heated at 55°C to decompose the water and methyl ester and rise the washing efficiency. The washing process is repeated three times as far as the pH value of methyl ester is decreased to 7. Finally, the methyl ester is heated at 100°C to remove any water and residue alcohol beneath the 200mmHg vaccum condition.
2.3. Experimental setup and procedure
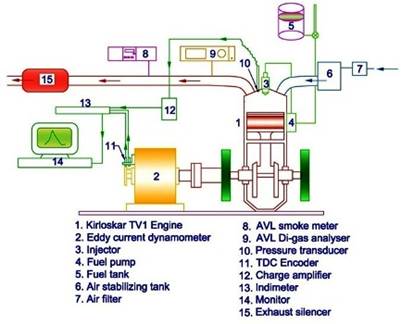
Experiments are accomplished in a single-cylinder, water-cooled, naturally aspirated direct injection diesel engine of 5.9 KW rated power spanned with an eddy current dynamometer and it is represented in Figure 1. An eddy current dynamometer spanned to the engine is adopted as a loading device. The specification of the dynamometer is given in Table 1. The engine has hemispherical combustion chamber, with overhead valve arrangements completed by push rods and it retains the constant speed of 1800 rpm at all load conditions.
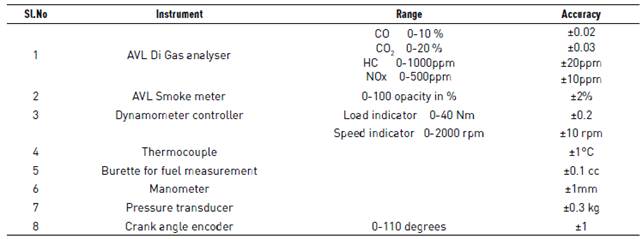
The speed of the engine is shown in the digital meter on the control panel. Engine cooling water inlet and outlet temperature are determined by a thermocouple. The water entailed for engine cooling is supplied from an overhead cooling water tank. The engine is loaded by proposing an electric current into the dynamometer. Fuel consumption is measured with the help of a data acquisition system and sensor. The Fuel consumption is divvied manually with the help of a digital stopwatch and burette. Exhaust emissions are measured with a NDIR (Non-Dispersive Infrared) based AVL Di Gas 444 gas analyser. The analyser afforded a CO computation range from 0 to 20% by volume with a resolution of 0.01%, NOx range of 0 to 5,000 ppm with an aspiration of 1 ppm and HC range of 0 to 20,000 ppm with a resolution of 1 ppm. The details of operating range, accuracy of the smoke meter and gas analyser are given in Table 2.
3. Results and discussions
In this section, oxides of nitrogen, carbon monoxide, hydrocarbon, smoke emission and brake thermal efficiency of tested antioxidants are discussed. Among the five concentrations tested, the best concentration with best antioxidant additive is examined.
3.1. Effect of oxides of nitrogen (NO x ) emissions
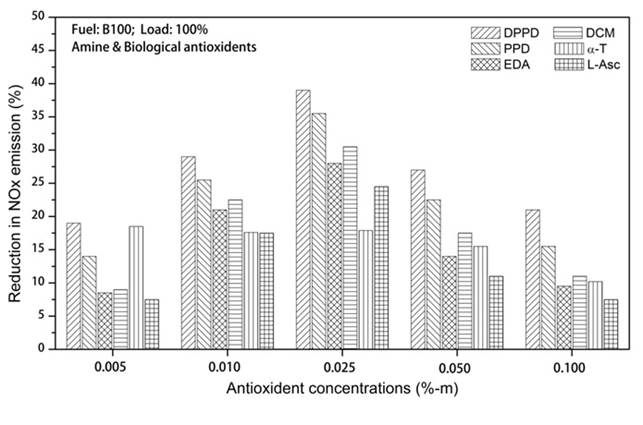
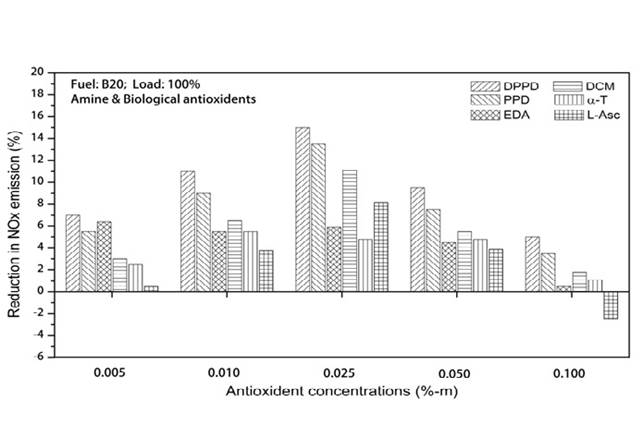
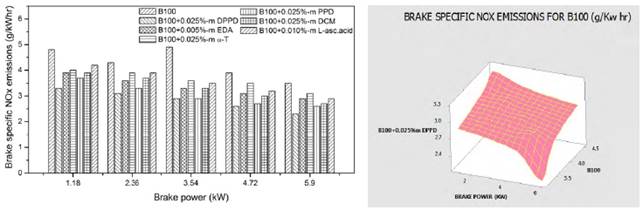
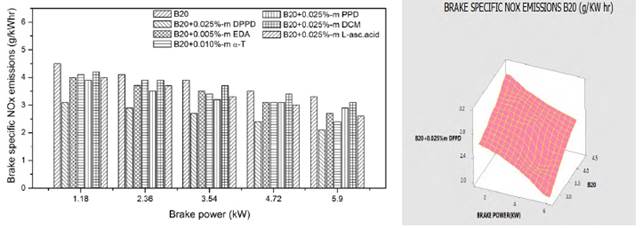
The impacts of amine and biological antioxidants on NOx reducing exertion for B100 and B20 fuels are shown in the Figures 2 and 3. Temperature plays predominant role in NOx formation. High temperature and high oxygen concentrations ensued in higher formation of NOx [14]. From Figure 2, at full load, the B100 fuel shows the NOx reducing activity as DPPD (39%), PPD (35.5%), DCM (30.5%), EDA (28%), L-asc.acid (24.5%) and α-T (17.9%) is the lowest. From Figure 3, for B20 fuel, we initiated the higher NOx reduction at DPPD (15.4%) followed by PPD (13.5%), DCM (11.1%), L-asc.acid (8.2%), EDA (6.4%) and α-T (5.5%) is found to be the lowest. DPPD is the most efficacious of the antioxidant examined, relinquished more than 15% decrease in divvied NO emissions at all engine loads. All the antioxidant additives reduced the NO emissions equal to 0.025%-m concentrations and tackle to decrease afar. The same result is perceived by Varatharajan and Cheralathan [15] and he contemplated maximum NO reduction for B20 fuel with DPPD additive is 9.35 for neat biodiesel it is 28.36 with 0.025%-m concentrations.
Similar trends are discerned by Dunn [16] and he perceived increased antioxidant agitation at lower loadings (below 1000 ppm) and constant or lowered NO agitation at higher loadings. The possible cause for the antithetical relationship between rate of treatment and quantity of NO reduction is that all the amine based antioxidants comprise nitrogen in its chemical structure and at higher loadings, the profusion antioxidant reacts in oxygen and too provoke NO. The concord of specific NO emission of mango seed methyl ester with the optimum antioxidant additive to B100 fuel is shown in the Figure 4 and for B20 fuel is shown in the Figure 5. For B100 fuel, the NO produced by DPPD additive and neat biodiesel at full load is 1.92 and 3.1 g/kwhr respectively. The corresponding NO emissions for B20 fuel are 1.73 and 2.12 g/kwhr consequently. Fenimore [17] presented the formation of NO through reactions of hydrocarbon radicals with molecular nitrogen and it is rendered in Eqs (1) to (4).
Brezinsky et al. [1] distinguished increased formation of CH radicals in biodiesel combustion. This could be the pre-eminent cognition for increased formation of NOx. Peroxyl radical (HO2) processed by ester is nearly five times more active than the alkyl peroxyl radical commenced by hydrocarbons. The highly reactive ester peroxyl radicals are devastated by them [18]. From the results, it is apparent that more free radicals are formed in biodiesel combustion and prompt NO could play a formidable role in biodiesel NOx emissions. Lin and Lind [19] constituted increased production of peroxyl radicals and reduced array of OH radicals in biodiesel combustion.
DPPD additive reacts with peroxyl radical to form primary amine radical and that is very reactive. Moreover, the peroxyl radical reacts with amine radical and compare benzoquinodiamine and nitroxy radical. These reacted products efficiently decoy the free radicals. Figure 4 indicated almost same NOx emissions to experimental work. There were five concentrations tested with B20 and B100 fuel. These fuels were mixed with 0.005%-m, 0.010%-m, 0.015%-m and 0.025%-m concentration of tested antioxidants and the best result was indicated in the figures with the best concentration.
3.2. Effects on CO emissions
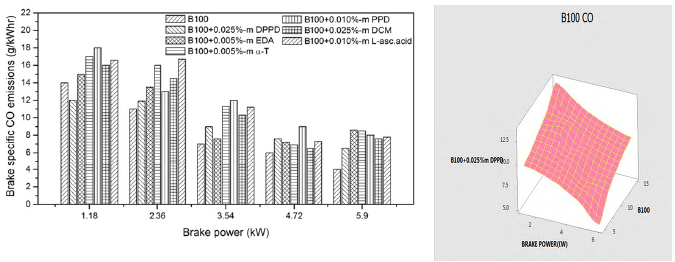
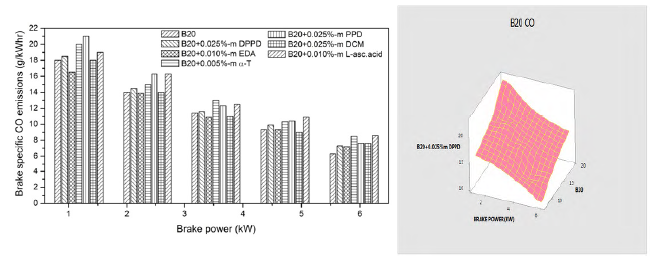
Figures 6 and 7 show the impact of amine and biological additives on brake specific CO emissions at various loads for B100 and B20 fuels. At full load, the DPPD additive has about 7.42% and 6.44% more CO emissions than the neat biodiesel and B20 fuel subsequently. Addition of antioxidant additives result in the increase of CO emissions in biodiesel, and that can be expounded by the additive reducing the oxidation capability of CO. But, peroxyl (HO2) and hydrogen peroxide (H2O2) radicals are formed during oxidation. Furthermore, these radicals are converted into hydroxyl (OH) radicals by consuming heat inside the combustion chamber. These OH radicals are mostly liable for the conversion of CO into CO2. This reduction in free radicals may have a remarkable influence on the formation of OH radicals and oxidation of CO and HC. Figures 6 and 7 represented the analysis of variance with respect to experimental work and the result was lower CO emissions than experimental work.
3.3. Effects on HC emissions
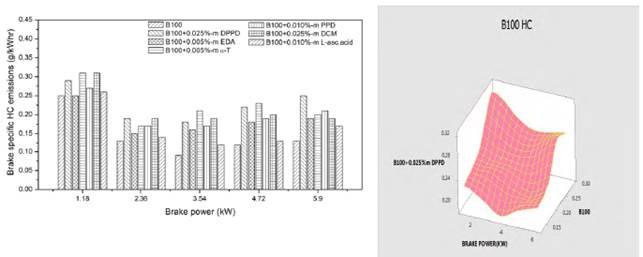
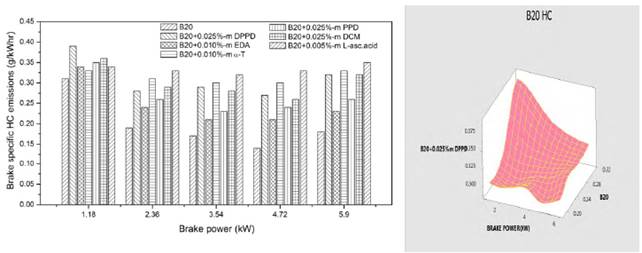
Figures 8 and 9 show the influence of amine and biological antioxidants on brake specific HC emissions at various loads for B100 and B20 fuels. Highest hydrocarbon emissions are recognized with Dichloromethane additive (0.235g/kwhr) for B100 fuel in Figure 8 and for B20 fuel it is 0.297g/kwhr in Figure 9. Lowest hydrocarbon emissions are inspected with ethylenediamine (0.194 g/kwhr) for B100 fuel in Figure 8 and for B20 it is 0.225g/kwhr at full load in Figure 9. It is found that addition of antioxidants led to increase in hydrocarbon emissions at full load. This increase in HC emissions could be owing to the reduction of oxidative free radical generation by antioxidants, and also the poor volatility of mixture and aromatic content which lowers the laity of combustion. Figures 8 and 9 indicated the individual representation of analysis of variance with respect to the experimental work.
3.4. Effects on smoke emissions
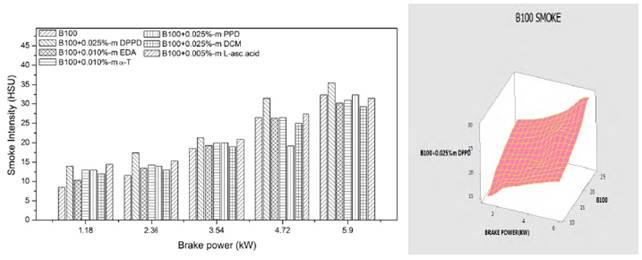
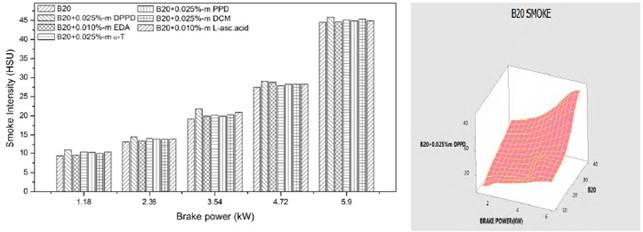
Figures 10 and 11 show the feature of the exhaust smoke emissions of B100 and B20 fuel containing the effective antioxidant additives. The results revealed that the smoke is almost persistent and imperceptible up to 2.36kw brake power and increased at high loads for all the blends. This is reasonable due to the fact that more fuel is combusted in the diffusion phase at high load than that of at low and intermediate load, leading to the increase of smoke emissions. The amine antioxidant DPPD additive produced lower smoke emissions out of the tested antioxidants and it was compared in analysis of variance in Figures 10 and 11.
The DPPD additive increased the smoke density by 14.5% and 8.9% for B20 and B100 fuels appropriately at full load. There are many factors bestow to the increase of smoke density with antioxidant addition. The possible causes for the increase of smoke are increase of C-C bonds, reduction of oxygen squeak and increase of aromatic placate owing to the addition of antioxidants with fuels.
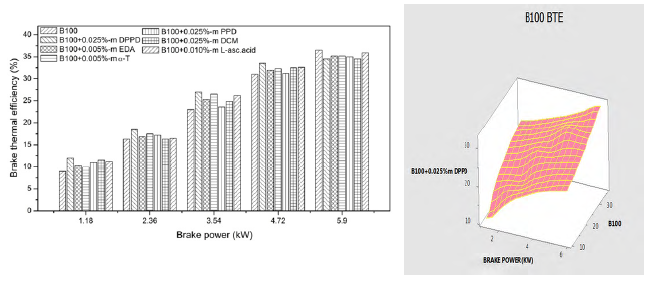
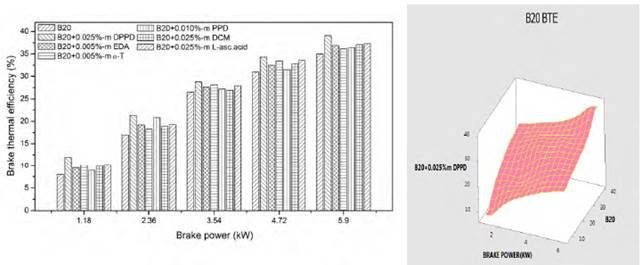
3.5. Effects on brake thermal efficiency
Figures 12 and 13 indicate the variation of brake thermal efficiency with brake power for B100 and B20 fuel. At part loads, changes in brake thermal efficiencies owing to antioxidant additions are apparent. But, at full load efficiencies are vaguely lower excluding the neat biodiesel in Figure 12. At full load, the brake thermal efficiency of DPPD with B20 fuel is 39.2%, but the base B20fuel brake thermal efficiency is 40.1%. The maximum brake thermal efficiency was obtained with DPPD additive with 0.025% molar concentration and the result was merely the same to the experimental with analysis of variance.
But, 0.91% increase in brake thermal efficiency is found with amine antioxidant DPPD. Addition of antioxidants to biodiesel fuels provocateur the reaction rate, and hence, engine brake thermal efficiency [20]. This increase in brake thermal efficiency is probably of the increased cylinder pressure with the addition of antioxidants. From the Figure 13, the experimental value is almost equal to the analysis of variance.
4. Conclusion
This paper has delineated the reputation of amine and biological antioxidants on NOx emissions powered by mango seed biodiesel in a direct injection diesel engine. The following denouements can be drawn hinged on the experimental results.
The antioxidant is effective in controlling NOx emissions. But, they have notably increased the HC, CO and smoke emissions.
Among the examined antioxidants DPPD observed to be efficacious for decreasing in NOx emissions thanDEA, PPD, DCM, α-T and L-ascorbic acid. DPPD presented 39% reduction of NOxin B100 fuel and 15.4% in B20 fuel from 0.025%-m concentration. It is detected that NOx reduction is in the order of DPPD>PPD>DCM>L-ascorbic acid>EDA> α-T.
It is found that 0.025%-m concentration exhibits the best reduction in NOx among the five concentrations tested. Prompt NO could be the major cognition for biodiesel NO x effect. It is concluded that amine based antioxidants reduce the NOx formation than biological antioxidants for the reason that, the amine based antioxidant DPPD efficiently ambushes the free radicals.
The DPPD additive increased the CO emissions over 7.42% for B100 fuel and 6.44% for B20 fuel at full load. The use of antioxidant additive with biodiesel fuels lead to a remarkable increase in HC emissions by the biological antioxidant dichloromethane (DCM) and it is owing to the reduction of oxidative free radical generation by antioxidants, and also the poor volatility of mixture and aromatic content which lowers the laity of combustion. For B100 fuel, DCM exhibits 0.235 g/kWhr for B100 fuel and 0.297 g/kWhr for B20 fuel. This is owing to the element that the use of antioxidants in biodiesel hindered the conversion of CO.
Smoke density is slightly increased with the addition of antioxidants in biodiesel. Brake thermal efficiency is found to be significant. Though, slight increase in brake thermal efficiency (0.91%) is deduced with antioxidant fuel mixture at full load.