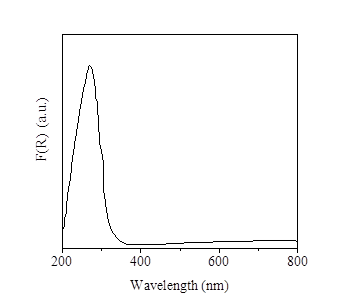1.Introduction
Mesoporous materials such as MCM-41, MCM-48, SBA-15 and KIT-6 are useful in several applications because of their pore network topology and adjustable pore size, controlled by the choice of surfactant agent and synthesis conditions. SBA-15 and KIT-6 have structures analogous to MCM-41 and MCM-48, respectively, but they possess a tridimensional pore network, composed of large diameter pores (5-15 nm) interconnected by channels of around 1.5 nm in diameter. During their synthesis, low cost surfactants are used, compared to the ionic surfactants used in the synthesis of the MCM materials [1,2]. The combination of high pore size and high surface area favor the application of these materials as supports in reactions with bulky molecules because mass transport limitation effects are minimized.
Limonene epoxide is used as an intermediate in the fine chemistry industry in the production of carvone [3] and biodegradable polymers [4]. It is possible to prepare it by catalytic epoxidation of limonene using Ti supported on mesoporous materials [5-10]. Using Ti-MCM-41, A conversion of 50% and selectivity to epoxide of around 60% after 7 h of reaction in acetonitrile as solvent was reported [5]; authors found that the use of Ti-MCM-41 increases the catalytic oxygen transfer process, avoiding the free radical mechanism that decreases the selectivity to limonene epoxide [5]. It was studied Ti-MCM-41 modified by silylation to decrease the hydrophilic character of the catalyst surface and adjusting the hydrophobicity of alkenes; the yield to limonene epoxide was 84% using ethyl acetate as solvent [7]. This study demonstrated that a post-synthesis modification by partial silylation of the surface (54%) is a good way to increase the limonene epoxide yield [7].
It was studied the epoxidation of limonene catalyzed by TS-1 zeolite and by Ti-SBA-15 [9]. Methanol was the solvent and hydrogen peroxide the oxidant; the catalytic activity was studied between 0 and 120 ºC with reaction times between 0.5 and 24 h. The selectivity over TS-1 reached 91% but at a very low conversion of limonene (2%). The conversion of limonene over Ti-SBA-15 was 5% and it did not change with temperature. The maximum selectivity was 68% (0 ºC, 0.5 h) [9]. In another study with TS-1 (Ti loading of 3.01 %w/w) and H2O2 (60% in water) [11], it was found that the highest selectivity to limonene epoxide was 35 %, with a high conversion of H2O2 (94 %) and limonene (60 %), and an oxidant efficiency of 64% after 10 days at 80 ºC. They used a limonene:H2O2 molar ratio of 1:1, 80% w/w of methanol and 3% w/w of TS-1. The oxygenated by-products detected were perillyl alcohol, carveol, carvone and 1,2-epoxylimonene diol. The catalyst was stable during three reuses.
Additionally, the oxidation of limonene with hydrogen peroxide (70% in H2O) was reported to be catalyzed by Al2O3 [12]. The catalyst was calcined at 450 °C during 15 h before reusing it, obtaining a conversion of limonene higher than 99% after 10 h and five recycles. The selectivity to limonene epoxide was 90% and the yield was 63% after 10 h at 80ºC (2.5 mmol limonene, 6 mmol H2O2 and 0.08 g Al2O3, ethyl acetate as solvent.).
Among Ti catalysts based on mesoporous materials, Ti-KIT-6 has not been reported for the epoxidation of limonene; Ti-KIT-6 has shown to be active for the liquid phase epoxidation of styrene, with yields higher than Ti-SBA-15 [13]; and selectivities up to 38% have been reported for cyclohexene epoxidation [14]. In this work, we report the activity of the catalyst Ti/KIT-6 for epoxidation of limonene in the liquid phase using TBHP as oxidant.
2.Experimental section
2.1 Synthesis and characterization of the catalyst
Synthesis of KIT-6 was carried out following the procedure reported in the literature [15]. Ti was incorporated by an impregnation method, yielding a Ti/KIT-6-PR material with a theoretical Ti/Si molar ratio of 13%. Briefly, 5 g of KIT-6 was stirred with 402.72 g of H2O and 16.96 g of HCl and cooled at 5 ºC; then pH was increased to 9 using concentrated NH4OH. Tetrapropylorthotitanate (TPOT, 97%, Aldrich) was used as Ti precursor; it was added to ligand acetylacetonate (ACAC, 98%, Aldrich) in 20 mL of anhydrous ethanol, and then were mixed with the KIT-6 suspension and stirred for 2 h. The solids were filtered and washed with 600 mL of ethanol to eliminate the superficial titanium.
The elemental analysis was carried out in an atomic absorption spectrophotometer Varian 220FS. The diffuse reflectance UV-vis in a Varian Cary 5000 spectrophotometer equipped with a Praying Mantis cell (Harrick Scientific). The nitrogen adsorption-desorption isotherms were determined in a Micromeritics ASAP 2010. The samples were degasified at 250 °C for 12 h prior analysis. The pore size distribution was obtained with the BJH model. The powder X-ray diffraction patterns at low angle was carried out in a Bruker D8 ADVANCED diffractometer.
2.2 Catalytic tests
In a typical reaction, vials of 2 mL were loaded with 1 mL of a limonene in acetonitrile solution (0.317 M), 43.2 μL of tert-butyl hydroperoxide (TBHP, 70%) and 20 mg of the catalyst. The temperature of the reaction (30, 50, 60 or 70 oC) was controlled with an oil bath, and magnetic stirring at 1000 rpm. At the end of the reaction, the catalyst was separated by centrifugation and aliquots of the reaction mixture (2 µL) were analyzed by triplicate using gas chromatography in an Agilent 7890N. We used an HP-5 capillary column (30 m) connected to a flame ionization detector (FID) and to a mass spectrometer. The oven heating program was: 110 ºC during 1 min and then 160 ºC for 3 min with a heating rate of 10ºC/min. Limonene and its epoxide were quantified through their respective calibration curves. The oxidizing agent content was determined after separation of the catalyst through a titration of 100 μL of the reaction mixture with a sodium sulfate solution (0.09821 M).
The leaching and reusing tests were carried out at 50 °C using acetonitrile as the solvent. For leaching tests, after 5 minutes of reaction, the catalyst was separated by centrifugation and the reaction was continued at 50 °C up to 7 h, and aliquots were analyzed at 1, 3, 5 and 7 h. Ti-KIT-6-PR was reused after 7 h of reaction. It was separated by centrifugation and dried at 100 °C before testing under typical reaction conditions. In some cases, the catalysts were calcined at 550 ºC for 5 h. In this case the heating rate was 1 ºC/min.
3.Results and discussion
3.1 Characterization of materials
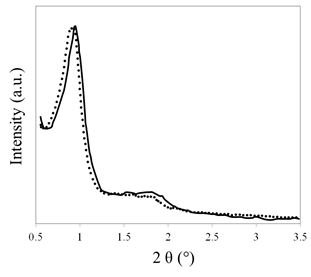
All diffraction patterns at low angle [Figure 1] exhibit a typical peak at 0.89° which corresponds to the (211) reflection of the cubic structure Ia3d, characteristic of KIT-6 mesoporous materials. This shows that the mesoporous structure of KIT-6 is not affected by the incorporation of Ti.
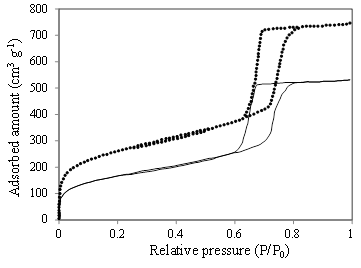
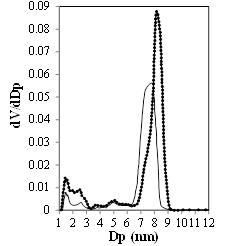
The N2 physisorption isotherms of KIT-6 and Ti-KIT-6-PR are typical type IV isotherms, with a hysteresis loop that corresponds to well-defined mesopores [Figure 2]. The surface area and pore volume are not affected by Ti incorporation, when reported per gram of KIT-6. There is a small decrease in average pore diameter, but the mesoporosity is maintained [Table 1; Figure 3].
The UV-Visible diffuse reflectance spectrum of Ti-KIT-6-PR shows an intense broad band centered at 267 nm [Figure 4], which has been assigned to Ti(IV) with a decreased tetrahedral character. There are shoulders at low energy that can indicate an effect on coordination by adsorption of water molecules or by incipient formation of oligomeric TiOx species; signals attributed to formation of anatase or other coordination states of Ti are not observed [16,17]. Ti4+ tetrahedral species on supported mesoporous materials MCM-41 and KIT-6 have been attributed as the active sites in in the epoxidation reaction of styrene and cyclohexane, respectively [14,17].
3.2 Catalytic tests
Our reaction results are summarized in Table 2. The blank reaction shows that a catalyst is required. Hydrogen peroxide is a stronger oxidant than TBHP at 30 °C, but its use decreases significantly the selectivity to limonene epoxide, forming a large fraction of side-products. We also observed that the conversion of limonene and selectivity to epoxide in the 50-70 ºC range were not very sensitive to temperature, but they were higher than the results at 30 ºC. The lack of temperature sensitivity could be caused by mass transfer limitations, an unexpected result for this type of mesoporous materials.
When the reaction time was increased, Table 3, the conversion of limonene and TBHP and the selectivity also increased, albeit slightly, an indication that the catalysts had not deactivated yet. An analysis of the time evolution of the reaction, reported in Figure 5, suggests that the main side products, carvone and carveol, are formed via the allylic oxidation route, whereas the diol is formed by the hydration of limonene epoxide; other side products were perillyl alcohol and p-mentha-2,8-dien-1-ol, the latter formed by isomerization of limonene epoxide. The selectivities calculated by normalization of areas were lower than those obtained by calibration curve [Table 3], suggesting possible differences in the response factors of the reaction products.
Table 2 Temperature effect on the epoxidation of limonene*
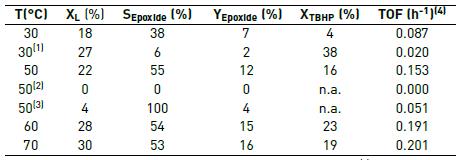
* 7 h, 1 mL of limonene solution in acetonitrile, limonene: TBHP = 1:1 (1) H2O2 as oxidant. (2) No catalyst. (3) Without TBHP. (4) TOF: Turn over frequency, mole of product per mole of Ti per unit time. n.a.: not analyzed.
Table 3 Effect of the reaction time on the epoxidation of limonene at 50 °C*
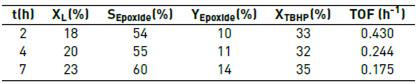
* 1 mL of limonene solution in acetonitrile, limonene:TBHP molar ratio of 1:1
The effect of the solvent upon the activity of the Ti-KIT-6-PR catalyst was also evaluated and reported in Table 4. The solvents used were acetone, ethanol, ethyl acetate, toluene, acetonitrile, and tetrathydrofuran. The best selectivity to limonene epoxide was obtained in the presence of toluene and acetonitrile, with the latter being the best solvent in terms of activity, followed by ethyl acetate. A similar trend was observed during the oxidation of limonene and styrene catalyzed by Ti-MCM-41 [16,17]. The literature suggests that acetonitrile does not form stable complexes with Ti atoms and therefore, its activity is high [17].
Turn over frequency (TOF) values were in the range 0.013 to 0.430 h-1, which are lower than those reported in the literature in similar systems for limonene epoxidation over Ti-MCM-41: 0.6-6.9 h-1 [10], 8.14 h-1 [5], and 34-60 h-1 [7].
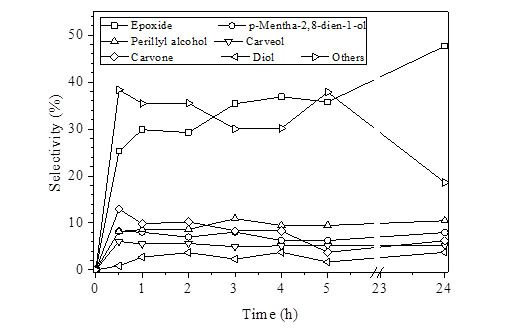
Figure 5 Distribution of the main products of the epoxidation of limonene. Reaction conditions: 1 mL of limonene solution in acetonitrile, limonene: TBHP molar ratio of 1:1, 50 °C. The selectivity was calculated by normalization of areas: %Selectivity = (Area of product)*100 /Σ(Area of products)
Table 4 Solvent effect on the epoxidation of limonene
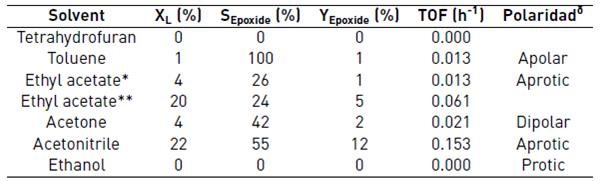
Reaction conditions: 7 h, 1 mL of limonene solution in acetonitrile, limonene: TBHP molar ratio of 1:1, 50 ºC. * 30 °C, ** 70 °C. δ Based on the solvatochromic parameter ET [18]
The Ti-KIT-6-PR did not leach active Ti species to the reaction media. Removal of the catalyst after 5 min of reaction kept the conversion constant at 7% when the reaction media was analyzed after 1, 3, 5 and 7 h. When the catalyst was reused under the best yield in Table 3, it was found that it was better to use high calcination at 550 °C instead of just drying at 100 °C to recover activity, presumably to remove adsorbed species. Yield after drying at 100 °C was reduced by 42%.
4.Conclusions
Catalytic tests using Ti/KIT-6 showed in all cases the formation of limonene epoxide, with TBHP as oxidizing agent instead of hydrogen peroxide. The highest conversion (23%) and selectivity to epoxide (60%) were obtained at 50 °C with acetonitrile as solvent and TBHP as oxidant. The catalyst does not leach active species, and can be reused after heating above 100ºC.