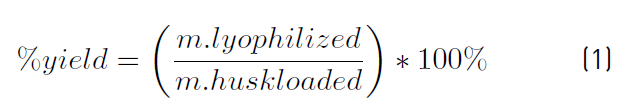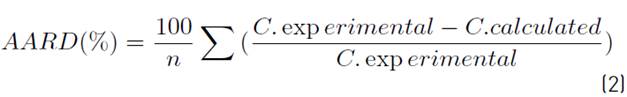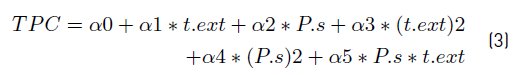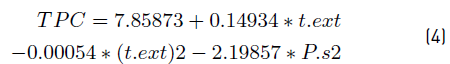1. Introduction
Today, it is possible to identify a crescent interest of consumers for products with a higher nutritional content. In that context, the supercritical fluids (SF) are being recognized as potential substitutes for conventional organic solvents for obtaining natural extracts. In this regard, numerous investigations have found that the SF are capable of providing natural extracts with a great bioactivity and quality, excluding any toxicity and responding to safety standards established for food, cosmetic and pharmaceutical industries [1, 2].
Between 2000 and 2013, more than 300 species have been evaluated to obtain extracts using SF to laboratory scale [3]. As an example, in Colombia, there are several studies that evaluate SFE to get products with added value from different vegetable matrices. All these studies are projecting with considerable success the supercritical fluid extraction (SFE) at a national level. However, Colombia has a wide availability of resources that have not been explored. In this sense, it is noted that Colombia is a tropical country with a high potential of production from the primary sector (first generation raw materials), where products such as coffee, palm oil, cotton and cocoa are traditional, and their processing generate elevated volumes of waste (second generation raw materials).
In particular, the cultivation of cocoa in Colombia registers a productivity higher than 47,000 tons per year [4]. Also in the world market, this fruit is commercially positioned in third place after sugar and coffee. Under this concept, the Federación Nacional de Cacaoteros (FEDECACAO) between 2002 and 2012 evaluated about 500 trees and from these selected 67 promising clones, among which the CCN-51 is highlighted (collection Castro Naranjal, variety ICS 95 * IMC 67, Ecuadorian origin) as one of the most productive not only in terms of the cocoa fruit but also wastes.
The productive chain of the cocoa in Colombia involves three steps: production, processing and marketing [5]. However, it is noted the absence of alternatives which promote the use of other components (mucilage, shell and husk), most of them being discarded and classified as vegetable waste. Considering that only the 25% of the total cacao weight is grain, in 2010 FEDECACAO estimated that the national generation of husk could reach a volume of about 30,000 tons [6]. Also at the global level, it is expected that each ton of dry grain will generate 10 tons of husk on wet basis [7]. Some studies mainly in Brazil, Ecuador, Malaysia and Spain have taken advantage of the content of dietary fiber and protein from the husk of cocoa for: i) the formulation of feed for animal consumption [8], ii) recovery of pectins [9] and sugars [10], and iii) the obtaining of adsorbent layers [11] to remove pollutants from water sources. However, the literature has reported limited information about obtaining extracts with nutritional and functional value from cocoa husk using SFE.
A supercritical extraction is defined as a non-conventional process, similar to leaching or solid-liquid extraction and it is characterized by working with solvents at conditions of temperature and pressure higher than their critical point [12]. As a solvent in this type of processes, it is common to use supercritical carbon dioxide (CO2-sc). This SF is inert and has a moderate critical point (Tc=304.2 K, Pc = 7.38 MPa). These properties make CO2-sc an ideal alternative for the processing of compounds with functional and nutraceutical value [13].
It is well known that the final product of any extraction is usually a liquid or solid fraction containing a significant number of compounds. Indeed, an extract is a mixture of all those components contained in an organic matrix (e.g. cocoa husk). It is necessary that those components present affinity with the solvent to reach successfully the extraction [3]. Therefore, the majority of studies related to SFE, tend to focus on defining conditions to obtain extracts with higher content of desired compound.
Nowadays, it is possible to identify a growing interest in obtaining extracts with high content of antioxidants, in particular polyphenols and carotenoids. Recently, it has been shown that cocoa, tea and wine are some foods with a high amount of polyphenols [14]. For this reason, products as cocoa have been recognized as a source of antioxidants due to the presence of polyphenols, especially flavonoids. In this case, flavonoids represent between 12-18% of cocoa bean polyphenols weight [15], being catechin and epicatechin, the most abundant in this fruit [16].
Regarding to carotenoids, those compounds are precursors of vitamin A, with a high antioxidant capacity being responsible of the pigmentation of several organic materials [17]. Since the cocoa bean is a source rich in polyphenols, it can be expected that other constituents of the fruit (husk, shell and mucilage) can be a considerable source of these compounds with an important functional and nutritional value. Among the main factors that must be evaluated in supercritical extraction process, the effect of temperature, pressure, co-solvent concentration, extraction time and particle size are the most prominent [13]. It is important to note that the power of solvation of all SF is influenced by its density; which is identified as a parameter dependent on temperature, pressure and polarity of the SF [18]. Temperature increases reduce the density of the SF, causing a significant reduction in the solubility of some components, which can be reflected as low extraction yields [13]. More regular testing temperatures oscillate between the 303.15 and 383.15 K [3]. However, when the goal is to extract bioactive compounds, it is necessary to be careful that temperature do not exceed usually the 333.15 K, to avoid thermal degradation[19]. On the other hand, high pressures generate significant increases in the density of the SF, improving their power of solvation and promoting the solubilization of the compounds of interest [20]. In most of the studies, the operating pressures vary between 10 and 80 MPa [3]. Since CO2-sc is one of the most used SF and knowing that it has a relatively low polarity, several studies have introduced the use of co-solvents in order to modify its solvation power and to promote the removal of polar compounds. In that case, the most widely used co-solvent is ethanol (in concentrations of less than 25%) [21]. This co-solvent has low toxicity, and can be removed easily from extracts, through atmospheric or vacuum evaporation.
Additionally, time and particle size are critical parameters in the extraction process. In this context, it is imperative to work with times of extraction that ensure interaction between the matrix and the SF, and at the same time, allow an adequate saturation of the fluid phase [22]. In this regard, the literature reports times ranging from 4 minutes to 6 hours [13]. In relation to particle size, it is common to experience between 0.10 and 3.0 mm for cocoa bean [23]. However, it is necessary to define an interval on which the SF could interact with all matrix surface area [24].
Regarding the antioxidant capacity of the polyphenolic compounds, the ORAC method has been widely used in the determination of antioxidant capacity of a lot of fruits and plants, including cocoa beans, for which it has been reported values between 450 to 1477 ET μmol / g ms [25, 26]. Notwithstanding the above, literature reports very little information about the antioxidant capacity of the main components of the cocoa fruit (husk, shell and mucilage). Taking into consideration the above, it is important to evaluate the factors involved in the process, in order to identify favorable conditions for the extraction of these metabolites. Accordingly, the objective of this work was to study and to correlate the influence of the extraction time, particle size, temperature, pressure and mass concentration of ethanol on total contents of polyphenols, flavan-3-ol and carotenoids from cocoa husk clone CCN-51, using CO2-sc + ethanol as solvents. Besides, it was decided to evaluate the antioxidant capacity of the extract obtained from the most favorable extraction conditions.
2. Materials and methods
2.1 Chemical reagents
Carbon dioxide (99.9% v / v) was taken from Linde Colombia SA (Bogota, Colombia). Absolute ethanol (99.9% v / v), methanol (99.9% v / v), gallic acid (99.9% w / w), (-) - epicatechin (99.0% w / w), β-carotene (99.9% w / w), vanillin (99.9% w / w), Folin-Ciocalteu reagent (99.9% v / v), sodium carbonate (99.9% w / w ), hydrochloric acid (1.2 N) from Merck (Darmstadt, Germany); and sodium hypochlorite (13% v / v) from Suquim Ltda. (Bucaramanga, Colombia). Trolox (99.9% w / w), and disodium fluorescein AAPH from Sigma-Aldrich (St. Louis, USA).
2.2 Raw material and chemical characterization
Raw material collecting
60 cocoa fruits of CCN-51 clone, were selected. Cocoa was collected from three farms in different regions of the department of Santander, Colombia: La Victoria (Rionegro, average height: 708 masl , average Temp: 298.15 K), Villa Monica (San Vicente de Chucurí, average height: 693 masl, average Temp: 300.15 K) and Lebrija (Lebrija, average height: 1086 masl, average Temp: 297.15 K). All fruits were collected from different trees, taking care to select those that had similar characteristics (average length of 22.13 cm, average diameter 9.26 cm, average weight of 965.28 g) and a homogeneous physiological maturity (24 weeks).
Preteatment of cocoa husk
Briefly, cocoa husk was washed and disinfected, using a sodium hypochlorite solution (0.05% v / v) latter, cocoa beans, pulp and mucilage were removed from husk manually. The husk recovered was subjected to a cutting process (pieces of about 2.00 ± 0.50 cm3) to reduce its size. Next, a heat treatment was applied to avoid possible oxidation and to inhibit the action of polyphenol oxidase enzyme [26]. In the first step of inhibition, approximately 1000 g of cocoa husk pieces placed in contact with 2000 cm3 of water at 368.15 (± 3.15) K in an enzymatic reactor for 5 min. In the second stage, the pieces were immersed in cold water to 274.15 (± 4.15) K for 20 min [27]. Drying was carried out at 323.15 (± 3) K using a stove with air recirculation system for 26 hours, where the material reached constant weight. Finally, the size of the material was reduced using a mill to reach a particle size of 0.258 mm.
Cocoa husk characterization
Physical Characterization
The physical characterization was based on the determination of average particle diameter using the method of the American Society of Association Executives (ASAE, 1996).
Chemical Characterization
The chemical characterization involved: i) assessment of moisture, ash, fat, fiber, protein and carbohydrates present in the material, using the methods 966.02; 923.03; 920.39; 920.87 and 962.09 of Official Analytical Chemists Association (18th Ed. AOAC, 1996), respectively. The extraction of polyphenols and flavan-3-ols was developed using the methodology developed in the research group [28]. The carotenoids content was measured using spectrophotometric techniques [29].
2.3 Analytical method
Determination of particle size and extraction time for polyphenol extraction
The influence of particle size (Ps) and extraction time (text) on the TPC, was evaluated using the factorial design center point + 22 + axial points centered on the faces, and it was analyzed using the response surface methodology [13, 23]. Three levels of particle size and extraction time were selected: mesh -50/+100 (~0.223 mm), mesh -16/+40 (~0.677 mm) and mesh -8/+14 (~1.785 mm); and 40, 120 and 200 min, respectively. The design was carried out at constant conditions of temperature (308.15 K), pressure (10 MPa) and mass concentration of ethanol (11% ethanol).
Extraction profiles of total polyphenols, flavan-3-ols and carotenoids
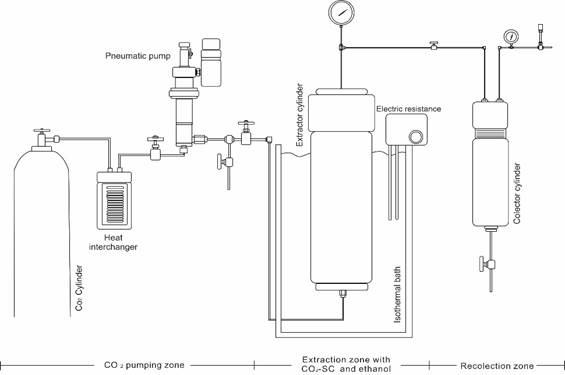
The behavior of the extraction profiles was evaluated in function of pressure, temperature and mass concentration of ethanol. Supercritical extractions were performed using a supercritical fluids lab unit [Figure 1] designed and built by the Instituto de Biotecnología y Agroindustria (IBA) of the Universidad Nacional de Colombia [30]. Briefly, a sample of 60.0 g of cocoa husk was placed into a 250 cm3 cylinder extractor. Ethanol was added manually (2, 11 and 20% w/w) over the mass of extraction. Then, the system was pressurized with carbon dioxide (up to 10, 15 and 20 MPa) using an air pump (Milton-Roy Williams, CP250V225 model). Cylinder temperature (308.15; 313.15 and 318.15 K) was regulated using an isothermal water bath with air bubbling. After this time, the system was depressurized and the extract was stored in a collector cylinder, equipped with ethanol, which worked as a solute trap. Finally, the extracts were collected in ambar vials, and storaged under darkness conditions at 276.15 K.
Chemical characterization of the collected extracts
The overall performance of each extraction was determined gravimetrically. The collected extracts were concentrated by vacuum evaporation (323.15 K and 0.01 MPa) and lyophilized (233.15 K and 0.013 MPa). Equation (1) was used to determine the extraction yield. It expresses the results as a percentage of lyophilized extract as gram per gram of cocoa husk dry basis (g LE/g db.).
Total polyphenol content determination:
In order to determine the TPC, 50 µl of sample was mixed with 1.5 ml of Folin Ciocalteu reagent (10% v/v) and incubated for 5 min under conditions of darkness. Then, 1.5 ml of a sodium carbonate solution (7.5% w/v) and the final mixture obtained were mixed and allowed for 60 minutes. The absorbance of samples was measured at 765 nm using distilled water as the blank. Finally, the TPC was determined using gallic acid as reference and expressed as milligrams of gallic acid equivalents per gram of lyophilized extract (EAG mg/g LE) [31].
Content of flavan-3-ols total (TFC) determination:
TFC was determined using the vanillin-HCl assay. 500 µl of the extract was mixed with 1250 µl assess vanillin (1% w/v, prepared daily in methanol) and 1250 µl of 9 M HCl in methanol. The mixture was incubated for 20 min at 303.15 K under darkness conditions. For each case, two blanks were prepared: i) replacing vanillin sample volume (1% w/v), and ii) replacing the methanol solution of vanillin. The final absorbance was determined at 500 nm subtracting the absorbance of the two blank. The TFC is calculated from a calibration curve constructed with (-)- epicatechin as standard and the results expressed in milligrams of epicatechin equivalents per gram of lyophilized extract (mg EP/g EL) [32].
Total carotenoid content (TCC)
Total carotenoid quantification was performed building a calibration curve of β-carotene using a spectrophotometer reading at 450 nm with ethanol as blank. Results were expressed in equivalent milligrams of β-carotene per gram of lyophilized extract (EBC mg / g) [33].
Determination of antioxidant capacity
The antioxidant capacity was evaluated with the hydrophilic ORAC. Briefly, 150 uL of fluorescein (FL) and 25 µl of the solution to evaluate (extract, standard or blank) were placed in the wells of a microplate with 96 dark places. The microplate was incubated at 310.15 K for 10 min and then, to each well 25 µL of AAPH were added. The fluorescence signal was evaluated 1 min each during 1 h under wavelengths of excitation and emission of 485 and 530 nm, respectively. is consistent with that observed in other works curve with Trolox as standard, and the results are expressed in micromol of Trolox equivalents per gram of lyophilized extract (μmol TE/g LE) [34].
Mathematical correlation of variables involved in the process of extraction
To describe mathematically the process of supercritical extraction for cocoa husk, a generic model is proposed in terms of each family of compounds (polyphenols, flavan-3-ols as well as total carotenoids) and efficiency. The dependent variables TPC, TFC, TCC and % Yield, and independent variables temperature (T), pressure (P) and mass concentration of ethanol (X) were correlated by implementing multiple regression matrix [35]. In all cases, both considered as quadratic nonlinear interaction of the independent variables (T, P, X, T * P, P * X, T * X, P2, T2, X2). The Calculation algorithm was programmed in MATLAB R2018b and the degree of adjustment of the proposed mathematical models were evaluated by calculating the average absolute relative deviation (AARD) expressed in the Equation (2):
n is the number of data, c experimental and c calculated correspond to the experimental and the model estimated value, respectively. The best regression is based on minimizing the error between the experimental value and the theoretical value obtaining values close to zero.
Statistical analysis
The influence of extraction time, particle size, temperature, pressure and mass concentration of ethanol was evaluated by variance analysis. The main effects plots, Pareto diagrams, Tukey HSD tests and other statistical analyzes were performed using Statistica7, developed by StatSoft Inc., on trial for Windows 7. The response surfaces were simulated using Matlab R2018b, MathWorks® developed.
3. Results and discussion
3.1 Characterization of Cocoa Husk
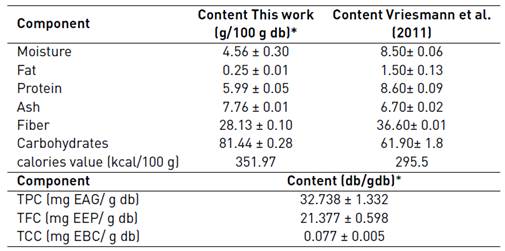
The profile of components quantity during the characterization of the for cocoa husk clone CCN-51 is summarized in the Table 1. The values of moisture, fat, protein, ash, fiber and carbohydrates were similar to those reported for other authors [8], that characterizes the husk of a Brazilian clone, with some differences, which could be attributed to several factors, as agronomic and environmental conditions and the physiological and genetic diversity [36].
*The results are expressed in terms of average and standard deviation for n=2
Related to the total polyphenol content (TPC), the value determined in this work was 1.56 times greater than reported in Ecuador [37] and 1.22 times lower than that quantified for husks of Brazilian clones [7]. Additionally, the TPC for cocoa husk obtained in this work (32.738 mg EAG/g db) was 3.53, 2.60 and 1.34 times higher than reported for the banana husk, avocado and passion fruit, respectively [38]. These results suggest that cocoa husk (agro-industrial waste), can be used as a source to obtain polyphenols. In the literature, there are not reports including contents of flavan-3-ols (TFC) and total carotenoids (TCC) for husk cocoa extracts. The values presented in Table 1 show that the TFC and TCC are of 21.377 mg EEP/g db and 0.077 mg EBC/g db, respectively.
3.2 Analysis of particle size and extraction time on total polyphenols content from cocoa husk
To determine the particle size (Ps) and extraction time (text) favorable to obtaining extracts rich in polyphenols using SCF, a surface response methodology (SRM) was implemented. The experimental data obtained (supplement data) were fitted to the second order expression which present in Equation (3).
In this expression, the relationship between the TPC and the linear and quadratic effect of the two independent variables (Ps and text) is considered. The Table 2 shows the estimated regression coefficients.
Table 2 Regression coefficients estimated from a second-order model
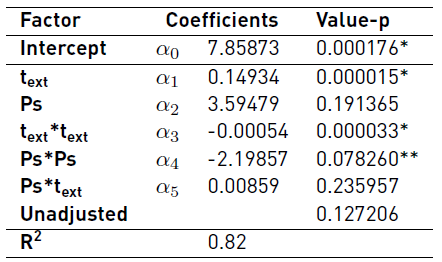
* Coefficients with significance (value-p<0.05) ** Coefficients with significance probability (value-p<0.1)
The results of statistical analysis using a confidence interval of 95% shows that lineal effect of text (α1) and quadratic effect of text (α3) are significant on response variable TPC. However, the results presented by of other researchers [13] show that the extraction time, size and morphology of the solid material have a direct effect on the amount of recovered extract. Therefore, in this work the confidence interval was modified to 90% to consider the effect of Ps on response variable TPC. Under this consideration, the quadratic effect of Ps (α4) was included into the fitted equation (Equation (4)].
The Equation (4) represents in 80.35% the behavior of the experimental data reported in the Figure 2 that shows the response surface obtained. Additionally, a routine programmed in Matlab R2018b was used to determine the maximum of Equation (3). The conditions for Ps and text that allows obtaining the maximal TPC were 1.105 mm and 147.18 min, respectively. However, it is important to underline that according to Tukey HSD test [Table 3], text and Ps have no significant effect on TPC after 120 min of extraction and for values less than 1.105 mm, respectively. Regarding to Ps, authors observed that Ps must be near 0.25 mm for different organic matrices [39, 40]. Therefore, for the subsequent analysis the text and Ps was fixed at 140 min and 0.26 mm, respectively. These values are considered enough to ensure adequate saturation of the fluid phase (CO2-sc+ethanol) and to facilitate the production of extracts rich in polyphenolic compounds from cocoa husk.
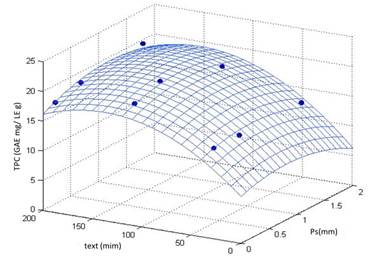
Figure 2 Response surface for TPC as function of particle size and extraction time. The results were obtained at 308.15 K, 10 MPa and X=11%
3.3 Analysis of temperature. pressure and co-solvent concentration on profiles of total polyphenols. flavan-3-ols and carotenoids
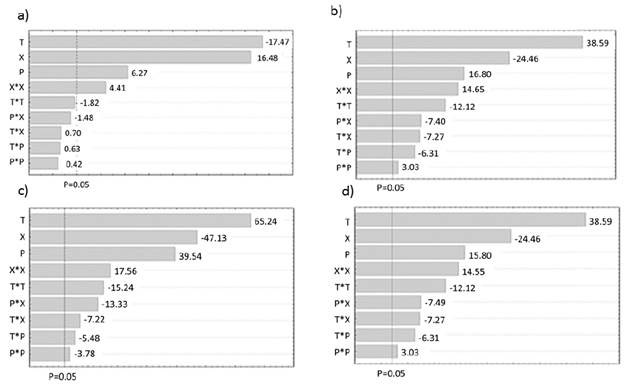
Figure 3 shows the TPC as function of independent variables evaluated (T, P and X). Error bars indicate the variation obtained in at least four measurements for TCP values. According to Tukey HSD test, the values with different letter show significant differences of 95% between average values determined for each experimental point.
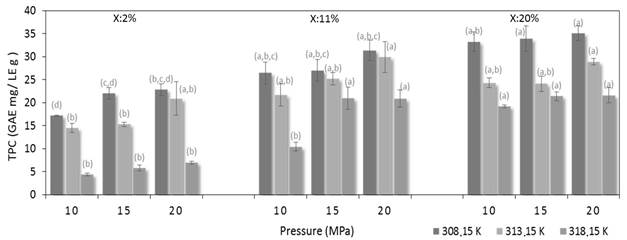
Figure 3 Variation of the total polyphenol content extracted as function of temperature pressure and mass concentration of ethanol
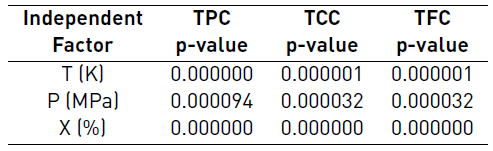
The ANOVA analysis present in the Table 4 shows that for the experimental conditions tested. the three independent factors (T. P and X) generate significant effects on the TPC, TFC and TCC (p-value <0.05).
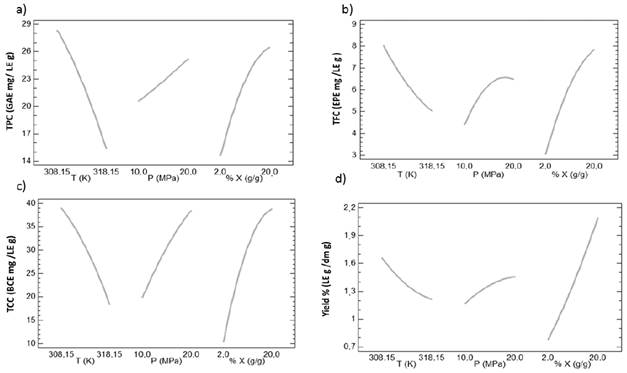
This can be confirmed graphically through Pareto diagrams presented in Figure 4. From this figure it can be concluded that the temperature is a factor with significant influence and also generates decreases on response variables. Additionally, the linear and quadratic effects of pressure and mass concentration of ethanol generates a significant increases on the response variable.
The main effects diagrams [Figure 5] were analyzed. As can be observed. the most pronounced changes occur with decreasing temperature and increasing the mass concentration of ethanol. producing increments until 53.84; 37.5; 25.0 and 31.8 % for the TPC, TFC, TCC and yield. respectively.
The behavior described in Figure 5 is consistent with that observed in other works [41-43], who made supercritical extraction from coffee husk, peach seeds and chontaduro pulp, respectively. In general, the results obtained in this study are particularly interesting because despite the existence of a polarity modifier (in this case ethanol). The apolar contribution of CO2-sc have a solvation power of the fluid phase. Therefore, this extraction technique allows the production of extracts rich in polar (polyphenols and flavan-3-ols) and nonpolar (carotenoids) compounds from cocoa husk.
In the range studied in this work, the most favorable conditions to obtain extracts rich in polyphenols corresponded to 308.15 K, 20 MPa and 20% ethanol. Where the TPC was 35.11 (±1.57) GAE mg/LE g. Also, for these conditions extracts showed to have a TFC and TCC 12.89 (±0.51) EPE mg /LE g and BCE 64.35 (±1.54) mg /LE g, respectively. Finally, the maximum TPC of this work is similar to that reported by Costa et al. [41] who obtained 36 (±1) GAE mg/LE g from coffee husk working at 323.14 K. 20 MPa and 8% ethanol.
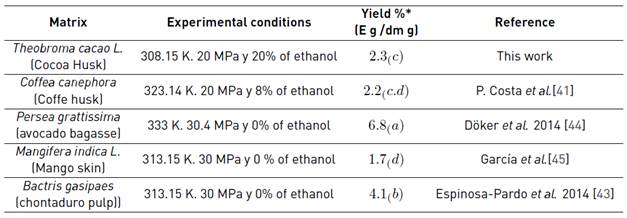
The maximum yield obtained was 2.3% which is considered according to typical values obtained by several authors in different organic wastes [Table 5].
3.4 Correlation of variables involved in supercritical extraction
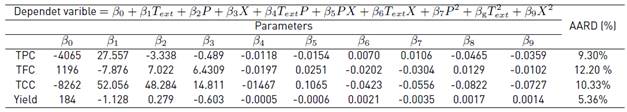
Table 6 shows the parameters and AARD (%) of the models proposed to describe the behavior of each of the dependent variables (TPC. TFC. TCC and yield) involved in the supercritical extraction from cocoa husk (clone CCN-51).
From the overall comparison of the values of AARD, it can be inferred that the proposed models are able to describe the global trend of the experimental data with values that do not exceed a AARD of 12.2%. At the time of predicting the behavior of extraction with SCF, it is common to work with values that are found in an interval of 1 % to 30 % of AARD [46]. Since, small changes in the independent variables bring big changes in the power of Solvation of the SCF.
3.5 Antioxidant capacity
The ORAC value for the TPC extraction under the most favorable conditions was 490 (±54) TE µmol/LE g. This value is 1.09 and 3.65 times higher than that reported for supercritical extracts of hibiscus (ET 450 ± 16 TE µmol / LE g) [47] and green Lavender (ET 134.06 ± 13.69 TE µmol/LE g) [41] respectively. On the other hand, it is noteworthy that this value is only 1.38 and 1.09 times lower than that reported by Martínez [48] for commercial synthetic antioxidants like BHT ( 678±10 TE µmol/LE g) and vitamin-E (536 ± 8 TE µmol/g vitamin-E). respectively. These results suggest that cocoa husk (clone CCN-51) is a potential source to antioxidant compounds extraction with a high aggregated value using CO2-sc and ethanol as solvents.
4. Conclusions
The influence of the main parameters of extraction using supercritical CO2 and ethanol in the obtaining of polyphenols and carotenoids from cocoa husk was studied. In that sense, extraction times higher than 120 min and particle size under 1.105 mm did not present significant effects on TPC. The conditions evaluated (T: 308.15; 313.15 and 318.15 K; P: 10; 15 and 20 MPa and X: 2; 11 and 20 %) showed that the TPC rises up to 53.84% with increases in pressure and mass concentration of ethanol. On the other hand, it can be expected that temperature generates reduction in TPC near to 48.28%. A similar behaviour in the variation of flavan-3-ols and total carotenoids content was observed. The supercritical fluid extraction of polyphenols, flavan-3-ols and total carotenoids was described through models that considered the linear and quadratic interaction effects between temperature, pressure and mass concentration of ethanol with an AARD less than 12.20%.
Finally, TPC and antioxidant capacity of the obtained extracts, are comparable to synthetic antioxidants and other compounds obtained from different sources. Consequently, the cocoa husk has the potential to be used as a source of bioactive compounds. This could represent the cocoa husk potential to obtain macronutrients and micronutrients that can be included in the production chain of cocoa. The above represents that the use of this natural resource provides economic and environmental benefits.