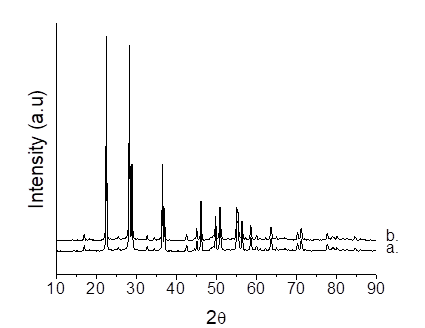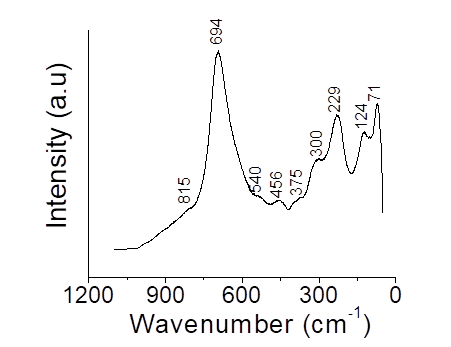1. Introduction
Oxidation is one of the most important synthetic routes for the conversion of chemical compounds into valuable intermediates and products for the chemical industry. During the last decade, the peroxo and hydroperoxo complexes with different transition metals, including W, V, Mo, Ti and Nb, has drawn attention due to their exceptional catalytic activity in the oxidation of alkenes, aliphatic and aromatic hydrocarbon compounds [1, 2]. However, most of these works take place via homogeneous catalysis [3-5], which makes the catalytic process more expensive and difficult to execute and separate the products and catalyst, especially at large scale [6, 7].
The use of an environmentally friendly oxidant such as aqueous hydrogen peroxide (H2O2), as a modifier on heterogeneous catalysts to make easily recyclable catalyst will be a challenging goal for the fine chemical industry. The catalytic technology target in this work using hydrogen peroxide as a surface modifier is developing large active sites, which can be applied for the oxidation of larger molecules. For niobia (Nb2O5) bulk, it is possible to improve the oxidizer properties just with a simple modification of Nb2O5 surface by treatment the solid with hydrogen peroxide (H2O2) [8-11]. The modified niobia shows high activity in the oxidation of cyclohexene [12], geraniol [13], and during the oxidation/dehydration of methanol to obtain dimethoxymethane [14]. In a previous work, we report the highly selective epoxidation of geraniol using Nb2O5/SiO2 and Nb2O5/MCM-41 as heterogeneous catalysts [15], now we intended to verify the formation of peroxo sites from of the surface modification of Nb2O5 with H2O2. This kind of surface modifications can produce a relative high concentration of peroxo groups on the surface [4, 16], this phenomenon occurs after a prolonged interaction between the niobia with the oxidant (H2O2) via a donor-acceptor mechanism to produce Nb5+δ- species on the Nb2O5 surface [17]. The peroxo groups are the catalytically active species responsible of the oxygen transfer from the catalyst until the substrate. However, peroxoniobate (Nb(O2)4)- compounds are formed at the same time, which can be isolated using alcohol solutions. In general, the formation of these homogeneous species can help to enhance the catalytic activity.
Olefins epoxidation is a widely-used transformation in the fine chemical industry. Epoxides are valuable building blocks and versatile commercial intermediates owing to the numerous reactions they may undergo. During the epoxidation reaction, it is important to eliminate the acidity of the catalytic material to control the selectivity towards epoxide. H2O2 is an attractive option as an oxidant that can epoxidize different compounds in the presence of various transition metal-containing catalysts, obtaining just water as a by-product. Another favorable advantage is the use of heterogeneous catalysts for easy separation from products and regeneration in some cases. Therefore, the development of heterogeneous catalytic systems for oxidation reactions using H2O2 as oxidant is highly demanded. In this aspect, the development of recyclable oxidation catalyst with high catalytic performance is a key issue.
The mode of the oxidant activation on the catalyst surface determines the selectivity and the formation of peroxo sites, these play an important role during the oxidation reactions [14]. In this paper, the oxidation of geraniol was examined over Nb2O5 treated previously with H2O2 for understanding the catalytic behavior and the nature of the active sites in Nb2O5.
2. Experimental section
2.1 Chemicals
The following compounds were used: Niobium (Nb2O5) (Sigma-Aldrich > 99.9 %), NH3.H2O (J.T. Baker, 37 %), H2O2 (Fluka, 30%), geraniol (Sigma-Aldrich > 98%) and methanol (Sigma, > 99.8%). All chemicals were high-grade products and used without further purification.
2.2 Catalysts preparation
The Nb2O5 was used without further treatment. A peroxoniobate Ammonium tetraperoxoniobate ((NH4)3 +[Nb(O2)4]-)) was synthesized using the procedure reported by [6]. The obtained solid was labelled as Nb2O5/H2O2. In a typical procedure, Nb2O5 (1 g) in distilled water (25 mL) with a 35 wt.% solution of H2O2 (25 ml) and ammonia (6 ml, 25 wt.% solution) were placed in a round bottom flask. The mixture was stirred for a few hours. When the solid was totally dissolved, acetone was added dropwise (100 ml) under stirring, a white precipitate was formed, which was filtered, washed with acetone and air-dried.
2.3 Catalysts characterization
The textural properties of the catalysts were determined by nitrogen adsorption at 77K using the conventional technique on Micromeritics ASAP 2020 equipment. The surface area was calculated using a multipoint Brunauer-Emmett-Teller (BET) model. The pore size distribution was obtained by BJH model, the total pore volume was estimated at a relative pressure of 0.99.
The XRD patterns were obtained on a PANalytical X-Pert-Pro diffractometer using Ni filtered and Cu kα radiation. Raman Spectra were obtained by Jobin-Yvon equipment model T64000 with a detector CCD. Spectra for each solid were taken over the range of 100 - 900 cm-1, scanning at a step size of 1.0 cm-1 with an integration time constant of 1 s.
The XPS data were obtained in a Thermo Scientific Escalab 250 XI spectrometer. Measurements were performed at room temperature with monochromatic Al Kα (hv = 1486.6 eV) radiation. The analyzer was operated at 25 eV pass energy and a step size of 0.05 eV. To ensures that the ambient oxygen does not interfere with the sample analysis, the solids were first degassed in a vacuum pre-chamber of 10-7 mbar, and then the work vacuum is adjusted in the analytical chamber at a value of 6.3 x 10-9 mbar. C 1s signal (284.6 eV) was used as internal energy reference in all the experiments. Determination of core-level peak positions was accomplished after background subtraction per Shirley using peak XPS software. Peaks in a spectrum were fitted by a combination of Gauss and Lorentz curves, which also allowed separating overlapping peaks.
2.4 Catalytic activity evaluation
Catalytic reactions were performed in a low-pressure glass reactor equipped with a magnetic bar. Nb2O5 or Nb2O5/H2O2 (10 mg) and H2O2 (30 %wt, 1 mmol) were placed into the reactor under N2 at 400 rpm for 2 h at room temperature. The inert atmosphere was necessary to avoid the presence of molecular oxygen as oxidant. Then geraniol, was added, the mixture was maintained for 4 h under nitrogen atmosphere under agitation. The reaction mixture (catalyst, geraniol and H2O2) was diluted with distinct solvents (2 ml), and then the solution was separated from the catalyst by simple filtration. The effect of geraniol concentration was studied in the concentration range of 0.25 to 3 mmol. The temperature was varied from 293 to 333 K and the Nb2O5/H2O2 weight from 4 to 10 mg. To prevent any mass-transfer resistance, the catalyst particle sizes around 100 μm and stirring rates from 200 to 600 rpm were used.
The reaction mixture quantification and identification were performed by a Varian 3800 gas chromatograph equipped with Saturn 200 mass detector and a capillary column β-DEX (30 mx 0.25 mm). The relative peak area of substrates and products using a normalization method determined the conversion and selectivity. For this, a comparison between elution times of the reaction products with authentic samples was performed.
3. Results and discussion
3.1 Catalyst characterization
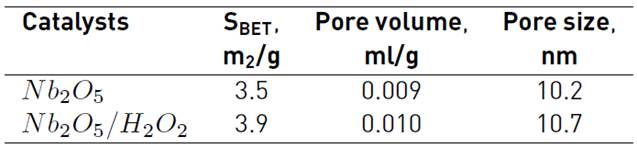
Surface area, pore size distribution and pore volume were determined from nitrogen adsorption/desorption isotherms (see Figure 1]. Figure 1 shows the isotherms obtained for Nb2O5 and Nb2O5/H2O2 catalysts, which are type IV with H1 desorption hysteresis loop. The late and steep adsorption step shows the relatively large pore size and narrow pore size distribution (PSD); this type of hysteresis loop is associated with structured porous materials, consisting of spherical cavities with fairly regular array, and narrow PSD. Table 1 details the textural parameters determined by N2 physisorption, including BET surface area, pore size and pore volume. Both samples show similar textural properties and no differences were observed after the treatment with H2O2 suggesting that the treatment with H2O2 does not have any effect on the morphological properties of the catalysts studied.
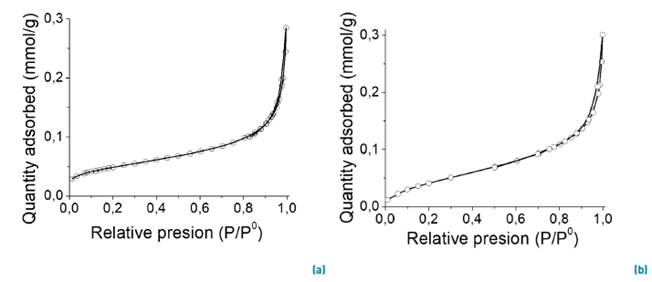
Figure 1 Nitrogen adsorption-desorption isotherms of solids: Nb2O5 bulk (left) and (b) Nb2O5/H2O2 (right)
The morphological features of Nb2O5 and Nb2O5/H2O2 catalysts are presented on Figure 2. X-ray diffraction (XRD) patterns of the catalysts confirmed the crystalline and well-defined structure of Nb2O5. The presence of Nb2O5 crystallites is confirmed for both samples showing diffraction peaks at 2θ = 22.5°, 28.5°, 36.7°, 46.1°, 50.7°, 55.2° and 56.1° assigned to the crystal planes (001), (100), (101) (002), (110), (102) and (111), respectively. It was also shown that the porosity and hexagonal structure of Nb2O5 and Nb2O5/H2O2 (T-Nb2O5, JCPDS card # 28-0317) catalysts remained intact after hydrogen peroxide treatment in agreement with N2 desorption/adsorption results (see Table 1]. No evidence was observed for a good match with the experimental and the theoretical powder diffraction patterns calculated by (18) for (NH4)3[Nb(O2)4] which was synthetized using the same H2O2 method. Although, peroxo species complexes are formed, they were not capable of modifying the structural and textural properties, which is in agreement with the N2-physisorption results.
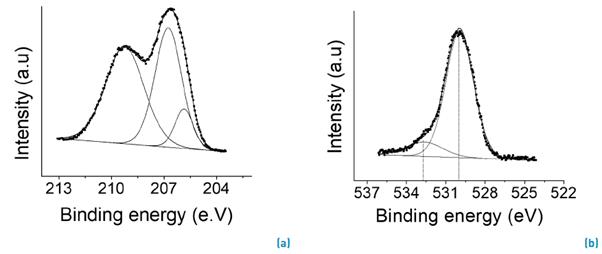
The surface composition of Nb2O5/H2O2 catalyst was determined by X-ray photoelectron spectroscopy (XPS). Figure 3a and 3b illustrate the deconvolution curves of O 1s and Nb 3d core levels. The Nb 3d spectra [Figure 3a) were deconvoluted using triplet lines attributed to Nb+5 phases [19, 20]. The binding energy of Nb 3d5/2 and Nb 3d3/2 lines was equal to 206.6 and 209.2 eV, respectively, with accuracy of ± 0.1 eV. The O 1s spectral line consisted of one doublet [Figure 3b) of different oxygen phases, attributed to oxygen atoms associated at two chemical bonds. The peak located at 529.9 eV corresponds to Nb-O bond and the peak close to 532 eV attributed to the formation of additional oxygenated groups on the surface of the catalyst. It is indicated that the peroxo group formation could be observed analyzing the region between 500 - 540 eV in the XPS spectrum [4, 15, 20]. They found that the peak around 532 eV was not observed for the commercial or synthetized Nb2O5 sample [4, 19]. There are two views about XPS of niobium oxides data in the literature. One group of authors claims that the peaks at 206 eV and at 205.4-205.6 eV correspond to 3d5/2 peaks of Nb+5 and Nb+4, respectively [21, 22], while another group assigns them to Nb+4 and Nb+3 [23, 24]. Our interpretation agrees with that of the first group. Based on the empirical relationship (∆(O-Nb)) between O 1s and Nb 3d5/2, it is possible to corroborate that the oxidation state of peroxoniobate is Nb+5 [25].
Raman spectroscopy was used to determine the vibration and rotation information in relation to the chemical bonds and symmetry in the Nb2O5/H2O2 structure, to find the finger print region of the spectra. In Figure 4, the Raman spectra of Nb2O5/H2O2 catalyst is shown. The spectrum displays the intensities of the Nb-O bending modes between 600-700 cm-1 corresponding to Nb2O5 structure. The broad peak detected at 694 cm-1 confirms the presence Nb2O5 in agreement with XRD analysis. A similar spectrum has been reported by [26]. They associated the broad band at 694 cm-1 with the formation of T-Nb2O5 phase. The T-Nb2O5 phase consists in 4×4 blocks that form the corner-shared octahedral of NbO6, each block is connected sharing the edges of the octahedron [27].
The high intensity observed in the band at 694 cm-1 for the Nb2O5/H2O2 catalyst, could be due to the extensive edge sharing of the octahedral. The bending vibrations at 540, 456 and 375 cm-1 are associated to distortions that may result in significant variations in the Nb-O lengths. Raman band between 300 and 150 cm-1 is due to the bending modes of Nb-O-Nb linkages [28] and the shoulder band at 815 cm-1 can be assigned to stretching vibrations of the peroxo groups [15, 18]. The peak at 229 cm-1 indicates the presence of Nb-OH bond, there were no other peaks noted, suggesting that no impurities were present.
3.2 Catalytic activity
Geraniol is an interesting substrate to be epoxidized to epoxy- or diepoxygeraniol. The selectivity to the desired epoxy must also be considered. In general, the epoxidation of geraniol occurs at the allylic double bond as was already observed by [29] and [13]. In this work, we used Nb2O5 and ammonium tetraperoxoniobate ((NH4)3 +[Nb(O2)4]-) labeled Nb2O5/H2O2. As can be seen from XRD, XPS and Raman spectroscopy, in the synthesis of this latter compound, the treatment of Nb2O5 with H2O2 did not modify the Nb2O5 structure and of peroxoniobate species [Nb(O2)4]- was not observed. The formation of ammonium tetraperoxoniobate ((NH4)3 +[Nb(O2)4]-) was not evidenced in this work, as indicated by XRD, XPS and Raman. However, Nb2O5 treated with H2O2 promotes the formation of peroxo species on the Nb2O5 surface, which may be related to its catalytic behavior. The new sites on this catalyst should favor the epoxidation reaction. Table 2 summarizes the results obtained for the catalytic activity, expressed in terms of conversion and selectivity to citral, allyl epoxide and others, which were the only products obtained in all cases. To corroborate if the epoxidation is going in the absence of catalyst, blank experiments (without catalyst) were carried out.
Table 2 Selectivity and activity obtained for the oxidation of geraniol over Nb2O5 and Nb2O5/H2O2 catalysts
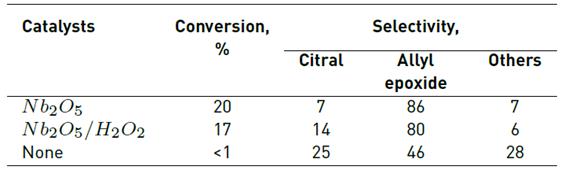
The main product obtained was allyl epoxide in all catalysts studied. No significant differences were observed in the catalytic activity using Nb2O5 treated with H2O2, which can be attributable to the amount of H2O2 used during the synthesis of Nb2O5/H2O2. The high selectivity at the allyl epoxide is due to the oxidation of the conjugated C=C double bond and the carbonyl function, respectively (see Figure 5]. The epoxide selectivity should be generated at peroxo sites and electronic structure assumptions, which is confirmed because the most nucleophilic group C=C double bond in the geraniol molecule is predominantly epoxidized [30], thus, the differences in selectivity observed is, at least in part, due to conversion «the higher the conversion, the higher epoxide selectivity», when is compared under the same reaction conditions. The other compounds are the sum of the other epoxide and glycols formed by the reaction between epoxides and H2O in the Lewis acid sites. Recently, Nowak and colleagues reported that the catalytic properties of niobium-based catalysts are very sensitive to the type of support used. In this study, the best result was 60% selectivity to all epoxides using Nb(Co)SBA-15 as catalyst [31].
To evaluate the effect of solvent during the product extraction, the oxidation of geraniol over the Nb2O5/H2O2 catalyst was tested in different solvents (see Table 3]. The conversion decreases as the dielectric constant of solvents decreases when acetonitrile, dichloromethane and dioxane were used as solvent, while using methanol, the conversion increases between 2 and 3 times compared with the other solvents. This phenomenon can be explained because methanol is capable to form hydrogen bonds, which increase the epoxide extraction obtaining a more efficient catalytic system.
Table 3 Conversion using different solvents for the oxidation of geraniol over Nb2O5/H2O2 catalyst
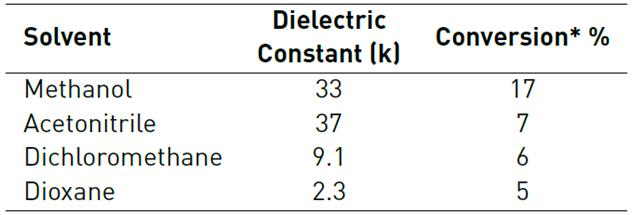
* After 4 h
The effect of the stirring speed in the range from 200 to 600 rpm was studied (not shown). In a typical profile, the initial rate increase as a function of stirring speed; however, no significant effect on the rate was observed at stirring rates over 600 rpm, indicating the absence of mass transfer resistance.
To determine the order of reaction, the results were expressed as log r 0 vs log(CGERANIOL). The initial rates were calculated using a third-order polynomial equation, during the consumption of geraniol with the reaction time. The dependence respect to geraniol (reaction order) varied from 0.8 to 1.1, but it depends on the temperature employed. It was reported that during the oxidation of benzyl alcohol over tungstic acid describes similar results, it was found a first-order dependence with respect of benzyl alcohol concentration (BzOH) and completely independent respect to the H2O2 concentration used [32].
The formation of peroxo sites occurs after prolonged contact of time between the niobium surfaces with H2O2. In addition, a high efficiency during the activation of these peroxo sites using H2O2 was observed during the progress of reaction. This effect is expected due to the reaction zero order obtained for H2O2, confirming that high amounts of H2O2 are necessary to lead the reaction.
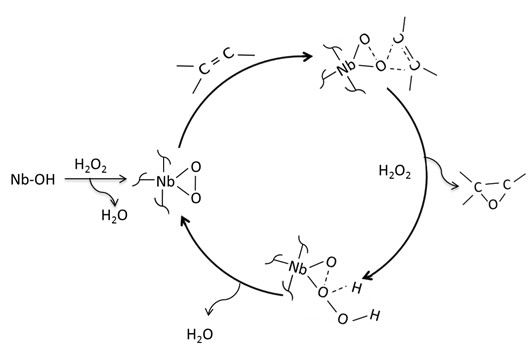
A proposed mechanism pathway for the oxidation of geraniol involves the activation and/or formation of peroxo sites by the reaction between H2O2 and Nb2O5/H2O2 (see Figure 5]. The nucleophilic allylic olefin group on geraniol molecule attacks the electrophilic oxygen of the Nb-peroxo group in a concerted oxygen-transfer step, forming a three-membered-ring transition state, which gives the allylic epoxide [33], as it is observed in Figure 5. In fact, a reaction order of one with respect to geraniol can consider the reaction between the peroxo sites and geraniol as the rate-controlling step.
The initial catalytic activity was recovered when the catalyst used was treated in the same way as is described in experimental part.
4. Conclusions
The activation of Nb2O5 catalyst with H2O2 favors the peroxo-sites formation more than peroxoniobate isolates species on the surface. The formation of peroxo sites is essential in Nb2O5 bulk for the geraniol epoxidation that is conducted by the formation of a three-membered-ring transition state that gives the good selectivity to the allylic epoxide. Surface modification is the key issue for controlling the product distribution in the oxidation of geraniol. No change in the textural and morphologic properties were evidenced by treating Nb2O5 with H2O2. The oxidation reaction is of first order with respect to the geraniol concentration and order zero with respect to H2O2 concentration.