1. Introduction
Phosphorus (P) and nitrogen (N) are the main nutrients causing eutrophication that lead to biodiversity loss and water quality problems [1, 2]. However, although an excess of phosphorus is problematic, modern agriculture is highly dependent on phosphorus fertilizer, which is derived from phosphate rock, a non-renewable resource with global reserves expected to be depleted within the following 50-100 years [3], making a strong problem for its contribution to agricultural and industrial development.
Both problems, phosphorus source depletion and eutrophication, can be tackled at the same time by the application of technologies aimed at recovering nutrients from wastewater streams [4]. For example, the centrate from anaerobic digesters in Wastewater Treatment Plants (WWTP), which is a N- and P-rich stream produced after sewage digestate dewatering, with concentrations between 750-1,500 mg N L-1 for nitrogen and 40-400 mgP L-1 for phosphorus compounds. Those figures are typical from WWTP using enhanced biological nutrient removal (EBNR) processes [5-8], which represents a good opportunity to recover nutrients from domestic wastewater. A technique proven to be a successful method to recover phosphorus from phosphorus-rich streams is the precipitation of struvite (MgNH4PO4.6H2O) [9]. Struvite is an effective, slow-release fertilizer that can be used to close the nutrient loop in between waste production and agriculture, as it simultaneously recovers N and P from waste effluents [10, 11].
Hence, struvite offers multiple benefits including limited negative impacts from agricultural runoffs into freshwater bodies, when compared with traditional chemical fertilizers. In fact, N losses have been reported to be considerably lower in struvite-treated soils than in soils receiving chemical fertilizers [10]. Struvite can also reduce nitrous oxide emissions and the need for phosphate rock, thus creating a more sustainable environment in line with a wider circular economy approach.
To date, various kinds of reactors have been developed at laboratory, pilot and full scales showing a great potential in recovering struvite from waste streams, among them are: fluidized bed reactor (FBR), air lift reactor and continuously stirred tank reactor (CSTR) [12, 13]. FBR is the most commonly used due to its ability to produce struvite pellets of large size, high crushing strength and purity [14]; however, its operation requires high flow rate and energy for mixing to ensure that the bed is continuously fluidized, so different efforts have been made to reduce energy demand and minimize losses of fine particles remaining in solution [15]. Guadie et al. [1] used a novel cone-inserted fluidized bed reactor for reduce unwanted crystal loss, while Le Corre [16] used stainless steel meshes to reduce the loses of fine particles in the effluent; Wang [17] conducted an economic analysis in FBR reactor using different Mg sources. On the other hand, in air-lift reactors air increases the pH by CO2 stripping and creates an internal recycle flow that allows crystals to grow until a critical particle size [18], however the costs of energy used in aeration can be considerably high. The stirred reactor or CSTR is one of the simplest and most effective technologies to produce struvite [19, 20], although the mixing force and the reactor hydrodynamics are significant factors that contribute to the formation of struvite crystals [21, 22].
In a stirred reactor, hydraulic shear forces can break struvite crystals into smaller fractions, which ultimately affect the resulting size of the precipitates [21]. These fine precipitates are more likely to be washed out with the effluent, decreasing the recovery of precipitates. Therefore, in order to optimize P-recovery efficiency after crystallization, it is beneficial to aim for larger particle sizes [20]. Additionally, larger struvite particles will have a longer effect on soils, increasing the absorption of nutrients from plants and crops [18].
The effect of stirring speed on struvite formation and on particle size has been little investigated. Liu [23] showed that mixing rates and contact time increase phosphorus removal efficiency, while Cerrillo [24] found that the maximum particle size decreased when the mixing speed was increased from 100 to 200 rpm. These results agree with the work reported by Kim [25] indicating that in struvite precipitation processes, removal patterns of nitrogen and phosphorus from the solution increased logarithmically as a response to the increment of Gtd values - i.e., the product between mixing intensity/velocity gradient (G) and mixing duration ( td ); however, the individual effect of G on particle size at speeds between 0 and 500 rpm is yet unknown. Therefore, it is important to evaluate the effect of stirring speed and mixing intensity in a wide range of velocity gradients (G) in order to optimize the amount of energy needed in these reactors to obtain high percentages of P and N recovery and larger particles sizes of struvite precipitates.
Thus, the main objective of this study was to evaluate the effect of stirring speed on struvite precipitation using the centrate from a conventional municipal WWTP. The removal of nutrients and the particle size were assessed to obtain the optimum stirring range for a struvite mixing reactor.
2. Materials and methods
2.1 WWTP
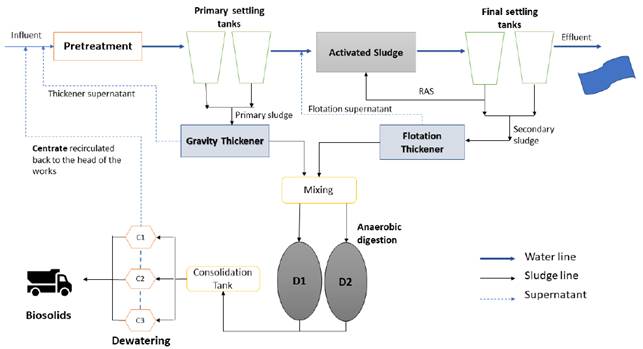
Centrate samples were collected in the municipal WWTP of La Llagosta, located in the Catalonia autonomous community, province of Barcelona, Spain, at 41 ° 30'12.62 "N and 2 ° 12'5.12" E [Figure 1], which has a treatment capacity of 43,000 m3d-1 and a population equivalent (p.e) of 358,000 inhabitants. This WWTP has a conventional system for the treatment of a blend of domestic and industrial wastewaters, which comprises pretreatment, primary settlement, and activated sludge units followed by a final secondary settling with chemical precipitation [Figure 2]. The process can remove 56% TKN and 86% TP for the raw municipal wastewater.
2.2 Analytical methods
Centrate samples were analyzed at IRTA facilities (Caldes de Montbui, Spain). Cations (Ca2+, Mg2+, K+) and anions (P-PO4 3-) concentrations were analyzed by ionic chromatography using a Metrohm 790 IC equipped with a Metrosep C4 150/4.0 column and a Metrohm 861 Advanced Compact IC equipped with a Metrosep A Supp 5-250 column and an 853 CO2 suppressor, respectively. Total solids (TS), total suspended solids (TSS), volatile solids (VS), NH4 +-N, TKN, TP and total alkalinity concentrations were determined according to Standard Methods [26]. The appearance of the precipitates was analyzed by using a scanning electron microscope (SEM, ZEISS EVO®MA 10) coupled to an energy dispersive X-ray microanalysis detector (EDX-Oxford, Inca) at Autonomous University of Barcelona (Cerdanyola, Spain). Meanwhile, the crystalline nature of precipitates was determined by X-ray diffraction (XRD, X'Pert Powder of Panalytical), using CuK-alpha radiation. The XRD patterns were recorded in the scanning range of 2-theta from 10° to 60°. Data were collected and processed using High-score-plus software. Identification of the phase peaks was accomplished by comparing the observed XRD patterns with a standard of struvite compiled by the the International Center for Diffraction Data (ICDD), the crystallography open database and the Powder Diffraction File (PDF) [27]. Crystal sizes were measured using ImageJ (1.51J8) software [28] by making 120 measurements of the crystals length for each sample.
2.3 Materials
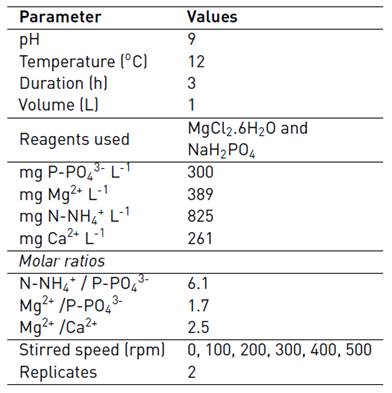
Due to the characteristics of the centrate, MgCl2·6H2O and NaH2PO4 were added to balance the P: N: Mg molar ratio to 1:6:1.7 (P = 300 mgP-PO4 3-/ L). This molar relationship was chosen because higher N:P molar ratio improves the precipitation of struvite instead of amorphous calcium and magnesium phosphates [29, 30], while Mg: Ca higher than 2:1 have the same effect [31]. Although struvite can precipitate at a wide range of pH values (7.0-11.5) [32], many investigations agree that the optimum pH value is around 9 (24, 33), for that reason NaOH in solution was added to reach pH 9. The principal conditions of the experiments are listed in Table 1.
2.4 Experimental setup
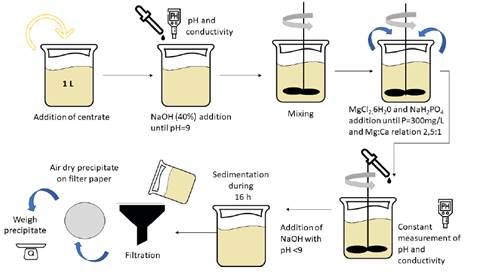
A standard jar tester with six paddles was used for struvite precipitation. Jars consist of 1L cylindrical glass vessels with 130 mm internal diameter. The flat paddles at the end of each stirrer shaft, which were made of stainless steel with a length of 7.6 cm and a height of 2.5 cm. The stirring device included a tachometer and a rev controller to adjust mixing rates ranging from 0 to 300 rpm. For the testing of mixing rates higher than 300 rpm, the same paddles were coupled to a laboratory stirrer. Mixing intensity (G) values tested in this study varied from 0 to 591 s-1. The applied G value was calculated based on the interaction between mixing rates of flat paddles and the corresponding velocity gradient based on a work previously reported [34], which found that the velocity gradient has a linear correlation to the impeller speed.
The applied mixing duration ( td ) was 3 hours in the six-stirring speeds tested (0, 100, 200, 300, 400 and 500 rpm) followed by 16 hours without stirring [Figure 3]. After 16 hours without stirring, the solutions were filtered using 1.2 µm membrane filters. In order to avoid struvite thermal decomposition that occurs above 55°C [35], the precipitates were air-dried at ambient temperature for 24 hours. Filters were weighed with and without the precipitate to calculate net struvite production; the TSS concentration from the centrate was also considered in order to calculate the total mass of struvite.
Considering that phosphate is the limiting reagent in this reaction, the maximum amount expected of struvite was calculated by multiplying the mmols of phosphate present in the centrate by the molecular weight of struvite.
3. Results and discussion
The characterization of centrate samples reported the following mean values: pH = 8.3; reactive phosphorus = 1 mgP-PO4 3- L-1; ammonium = 825 mgN-NH4 + L-1; calcium = 261 mgCa+2 L-1; total suspended solids = 116 mg TSS L-1; and total alkalinity = 3.1 gCaCO3 L-1.
Struvite was obtained even under no stirring conditions, but the total amount recovered increased with the increment in stirring rates. Higher removal of NH4 + was found as a result of increasing stirring speeds [Figure 4 and Table 2], which could be due to NH3 volatilisation. This did not affect struvite formation since the recovered struvite precipitate was between 89 and 112% of the maximum amount calculated from the composition of centrate samples [Figure 4]. In contrast, the Mg2+ removal increased between 0 and 100 rpm but later decreased as the stirring speeds increased, being slightly higher than the maximum that could be achieved by struvite precipitation [Figure 4]. In this case, high Mg2+ removals at different speeds may be due to the precipitation of other Mg precipitates, such as Mg(OH)2 since the excess mass of external magnesium source can rapidly react with OH- ions in the solutions to produce these amorphous products [25].
Table 2 Removal efficiencies (%), struvite similarity score XRD (%) and SEM-EDX results
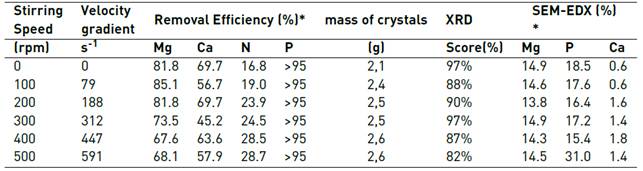
*regarding the initial content in the soluble phase; ** percentage of total atoms
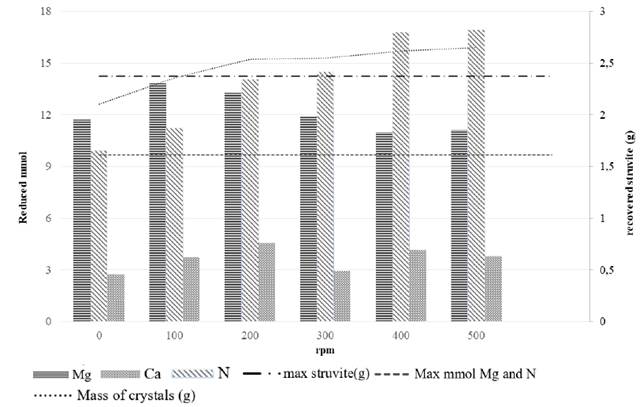
Figure 4 Reduced Mg, Ca and N (mmol) and recovered crystals depending on the stirring speed-velocity gradient
The Ca2+, Mg2+ and N removal from the soluble phase was between 2.73 and 4.55 mmol Ca2+, between 11.0 and 13.8 mmol Mg2+ and between 9.9 and 16.9 mmol N-NH4 +. The Ca2+ removal may be due to the formation of calcium carbonates like calcite (CaCO3) and monohydrocalcite (CaCO3·H2O), more favoured in comparison with the formation of calcium phosphate [36, 29].
As reported in Figure 3, there was no direct relationship between the removal of Mg2+ and Ca2+. This is because the initial high magnesium content enhances the phosphate reaction with Mg2+ rather than Ca2+ [37], thereby reducing the interference due to the formation of Mg2+ and Ca2+ precipitates such as dolomite (CaMg(CO3)2) and favours the formation of struvite.
High stoichiometric NH4 +: PO4 3- ratio (≈ 4.7) favor the struvite precipitation [36]; however, the mass of crystals above the maximum expected for struvite at stirred speeds between 200 rpm and 500 rpm [Figure 3] may be due to the formation of other precipitates not detected in the XRD, since the agitation possibly favours other reactions.
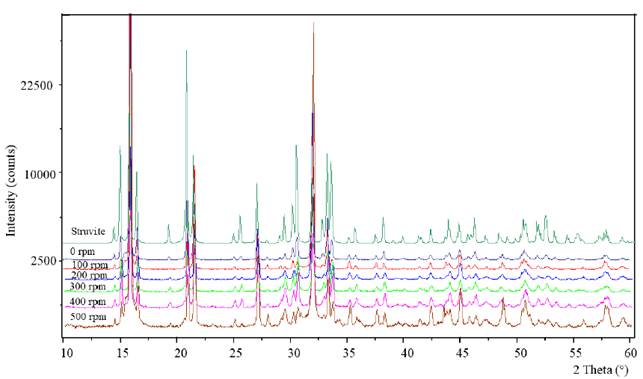
Despite finding greater removal of N and Mg2+ than the maximum expected by struvite precipitation, it was found by X-ray diffraction that the main product obtained at all stirred speeds was struvite, finding similarities between 82 and 97% with the standard of struvite (Score in Table 2 and Figure 5]. The SEM-EDX analyses confirmed the presence of Mg and P in the precipitates, with a minimum presence of Ca [Table 2 and Figure 6].
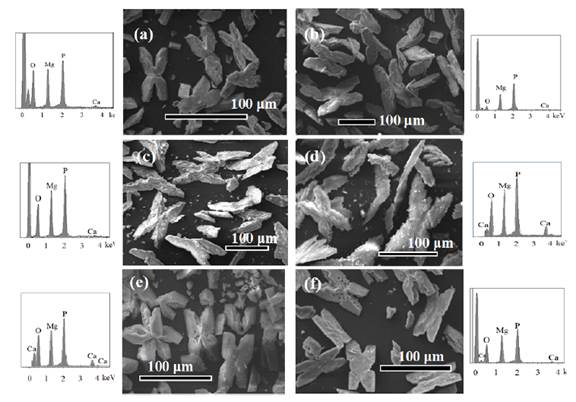
Figure 6 SEM images and EDS analysis of struvite crystals obtained working with different stirring speed: (a) 0 rpm, (b)100 rpm, (c) 200 rpm, (d) 300 rpm, (e) 400 rpm and (f) 500 rpm
Although Pastor [33] concluded that the degree of agitation of the system can exert a considerable influence on the crystalline habit (external appearance of the crystals), similar crystal shapes were observed in this work - i.e., stars, X shapes and needles [Figure 6]. The last shape, needles, are the most reported in by researchers, including Crutchik et al. [22].
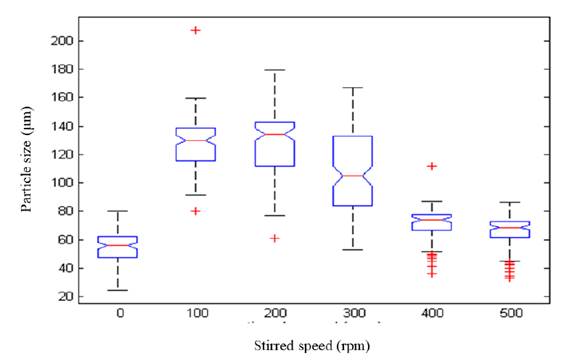
The attained data in this work confirmed the strong influence of stirring speeds on particle size distribution. The largest mean particle size was found at 200 rpm (128 µm; Figure 7] and the smallest size at 0 rpm (55 µm). According to t-student tests, there were no statistically significant differences between the particle size of 100 and 200 rpm (p >0.05); however, there were statistically significant differences between the particle size of 0 and 100 rpm, and 100 and 500 rpm (p<0.05). Therefore, the largest particle sizes were between 100 and 200 rpm, which corresponds to a G value between 79 and 188 s-1. According to Capdevielle et al.[30], high mixing rates have a negative impact on struvite crystal sizes due to struvite dissolution and crystal break, thus, although the mixing effect enhances mass transfer rates [25] and therefore the particle size, stirring speeds higher than 200 rpm were detrimental to the struvite crystallisation process.
4. Conclusions
The struvite formation is affected by the stirring speed. The largest mean particle size of 128 µm was obtained at 200 rpm - i.e, equivalent to a G of 188 s-1. The particle size of struvite crystals decreased with higher stirring speeds. Although the velocity gradient improves mass transfer rates of ions in the solution to support better crystal growth, high G values beyond the optimal can revert this effect, reducing the size of crystals formed or disfavoring their growth rates.
The P removal was not affected by stirring speeds, while the N removal increased with stirring speed achieving at 500 rpm a removal of 7 mmol more than the maximum possible by the sole formation of struvite. In this case, the agitation may favor the volatilization of N in the form of NH3 [38]. Although this did not affect the formation of struvite by the high contents of N-NH4 + in the centrate, it implied an increased emission and a potentially higher environmental risk.
Although the removal of Mg appears to be influenced by the stirring speed, the precipitation of other salts was minimal compared to the struvite precipitation, according to XRD. Therefore, a stirring speed range between 100 and 200 rpm (79 s-1 < G < 188 s-1) is recommended for struvite crystallisation with the benefit of producing large crystals, reducing energy consumption in stirring tanks, and reducing the volatilization of ammonia nitrogen.