1. Introduction
The importance of tissue engineering is growing due to the limited supply of donors and the desire to find better treatments to improve life quality [1]. Tissue engineering needs biocompatible, as well as biodegradable, mechanically resistant, and versatile materials in order to build three-dimensional structures that support cell growth, known as scaffolds [2, 3].
Both, natural and synthetic polymers have been widely used for this purpose. Silk is a natural polymer produced by worms, spiders and, nowadays, also by genetically modified mammalians [4]. Silk from Bombyx mori silkworm cocoon is mainly composed of silk fibroin (SF) and silk sericin (SS). A filament core of SF, predominantly hydrophobic protein, contributes with structural features and mechanical properties of silk; while SS as a glue-like hydrophilic protein, plays the role joining the filaments [4-6]. Although both SF and SS present adequate results in cell proliferation assays, SS has been the cause of adverse immune response related with silk used in some medical applications [4]. Furthermore, sericin-free SF has excellent mechanical properties, good biocompatibility and hemocompatibility, water-based processing, slower biodegradability than other natural polymers and high surface reactivity; all suitable properties for biotechnological or biomedical applications [1, 2, 4, 5].
Even for many industrial uses, SS is retired from silk by a process known like degumming. In textile industries, degumming is carried out in boiling water or detergent solutions [5]. In laboratory processes, it has been performed using sodium carbonate solution [6]. Fiber or filament of degummed SF is not the most versatile form of this material. In the search of a fibroin solution (FS) useful to produce scaffolds, biofilms, and other applications, several dissolving solutions of dehydrated calcium chloride or lithium bromide (LiBr) have been frequently used [2, 6, 7]. However, price is an important factor when it comes to choosing between them. Lithium bromide is 2.3 times more expensive than calcium chloride and dissolution results are quite similar, showing that calcium chloride use makes the process more financially efficient. Also, there are some relevant toxicity concerns when using lithium bromide. Even though lithium is an essential element for the human body [8] and has been used as part of treatments for some psychiatric disorders, its use was suspended in those treatments due to toxic effects on patients [9]. Regarding their use in the SF dissolution, both calcium chloride and lithium bromide required a subsequent dialysis process that is supposed to wash them off from the SF solution. However, it is highly important to prevent and control any traces of salt that might affect the solution purity or cause any rejection from the tissue.
Purification processes must be applied to remove residual salts from degumming and dissolving processes. For that purpose, dialysis tubes are commonly used against deionized water, with specific pore size that lets salts leave the solution, retaining proteins [2, 6, 10]. Usually cellulose tubes with a MWCO between 12 to 15 kDa are used to dialyze. These tubes, however, are expensive (from USD 100 up to USD 250 for 100 ft) [11] and increased the overall cost of silk solution production.
In the present study, the aim was to evaluate the efficacy of three different degumming methods to obtain silk fibroin; Raman spectroscopy was used for evaluating the obtained protein. Additionally, some different porous membranes were tested to compare their effectivity in the process, for research a low-cost method.
2. Materials and methods
2.1 Degumming of Silk
Bombyx mori cocoons were supplied by the Parque Nacional de la Cultura Agropecuaria (PANACA) based at Quindío, Colombia. Preparation of cocoons started by cutting them into small pieces, in order to increase the surface area. There were three different degumming methods, all using 1:100 silk:solution ratio, in magnetic stirring at 80 °C for 1 h: I) with distilled water (1:100 silk:water ratio), II) with distilled water and liquid neutral detergent (3:4 silk:detergent ratio) and III) with distilled water and sodium carbonate (10:1 silk:Na2CO3 ratio). After the degumming process silk was rinsed several times with distilled water.
2.2 Dissolution of SF
After the degumming process, the dry fibers were added to calcium chloride solution (1:20 fibers:solution) in a molar ratio of 1:2:8 of CaCl2/ethanol/distilled water [12]. The process was conducted at 55 °C for 1 h, under continuous magnetic agitation.
2.3 Dialysis of the solution of SF
The obtained solution was disposed in three different types of porous membranes: dialysis tubing (MWCO cellulose acetate membrane 12,000-14,000 Da), reused dialysis tubing (MWCO cellulose acetate membrane 12,000-14,000 Da) and food casing (sausage wrapping cellulose membrane). The dialysis process was carried out against distilled water for 5 and 7 days. The water was changed constantly to remove salts remaining in the dissolution process.
2.4 Raman spectroscopy of silk fibroin fibers
FT-Raman spectra of fibers degummed by each method was obtained with a Horiba Labram HRa spectrometer at a wavelength of 632 nm. Each sample was mounted on a slide, and the spectrum was acquired from 800 to 1,800 cm-1 for about 3 min per sample.
2.5 Conductivity measurements
Efficiency of salt extraction was evaluated through conductivity measurement before and after the dialysis process by using a WTW Teracon electronic conductometer.
2.6 Energy-dispersive X ray spectroscopy EDS
The dialyzed solution was then lyophilized by using a FreeZone plus 2.5L lyophilizer in order to obtain samples with a morphology that facilitate the analysis. This was accomplished via an EDAX EDS system attached to a JEOL-JSM 6,490 LV scanning electron microscope, allowing the proper identification of the presence of reagents remnants used both in preparation and dissolution.
3. Results and discussion
3.1 Raman spectroscopy
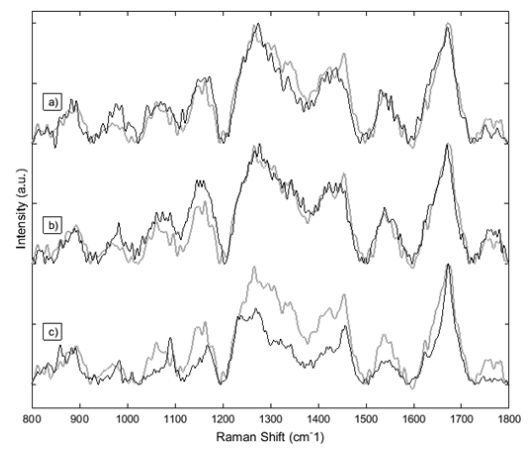
Figure 1 shows Raman spectra of fibers obtained with three degumming methods compared untreated silk (clear line). The first two methods, using distilled water and neutral detergent, show very few changes compared with the untreated material; this might indicate an incomplete or unsuccessful process of separation [13]. On the other hand, the spectra found for the fibers degummed with sodium carbonate shows several changes with untreated silk. Some of the characteristic bands of silk fibroin were present on the spectra, as was expected, giving some ideas of protein conformation [14]. An overall look at the spectra reveals peaks around 1,667 cm-1, 1,450 cm-1, 1,263 cm-1, 1,231 cm-1, 1,085 cm-1 and 854 cm-1 corresponding to structures presented in other silk fibroin fibers extracted from Bombyx mori. Amide I (1,700-1,500 cm−1) is present in all three samples regardless of the separation method. On the other hand, amide III (1,228-1,265 cm-1) band, often related with the content of β-sheet structures, in the SF treated with sodium carbonate is the only one that presented two peaks around 1,230 and 1,260 cm-1, leading to affirm that this treatment had a stronger effect on the structure of the protein by increasing beta sheet crystallites. Sericin-related peaks (1,155 and 1,525 cm-1) in I and II degumming methods, showed that separation of the proteins may not be fully completed compared to the sodium carbonate method; which showed a change in the 1155 cm-1 peak position and a significant downsizing in intensity for the 1,525 cm-1 peak [15-18].
3.2. Conductivity measurements
The electrical conductivity of a solution is essentially given by the concentration and mobility of the ions which it contains, since they are electric current transporters [19-21].
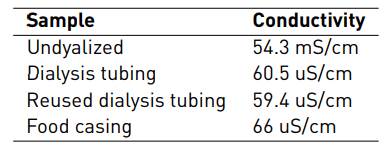
Conductivity measurements were executed after the solutions were dialyzed, taking the undialyzed one as the control sample, as a method to evaluate the dialysis efficiency. A lower conductivity measurement implies a lower ion concentration of the salts in the solution.
In Table 1, conductivity values for the dialyzed solutions with different membranes are displayed. It can be said that the reused dialysis tubing and the food casing showed appropriate results, which are not significantly distant from the displayed value of the ideal new dialysis tubing. In all three cases, there was a significant reduction of conductivity measures even up to a magnitude of order. This fact is associated with a suitable removal of the existing salts in the dissolution process. The literature does not show reports about the use of these materials for dialysis (reused dialysis tubing and food casing). However, this is an advantage, due to the possibility of performing efficient dialysis with cheaper materials. As the price of the dialysis tubing is considerably higher than the food casing, it becomes a good alternative due to price and availability. Regarding its use in the food industry, these kinds of membranes must fulfill some requirements in order to comply with international laws and regulations which look to guarantee consumer safety [22, 23]. The particle migration from the casing to the food must be within certain limits in order to assure the absence of alteration to the food product that can be harmful for the final consumer [24]. For this reason, it can be used for treating biomaterials without compromising its purity, non-toxicity and biocompatibility.
3.3 Energy-dispersive X ray spectroscopy EDS
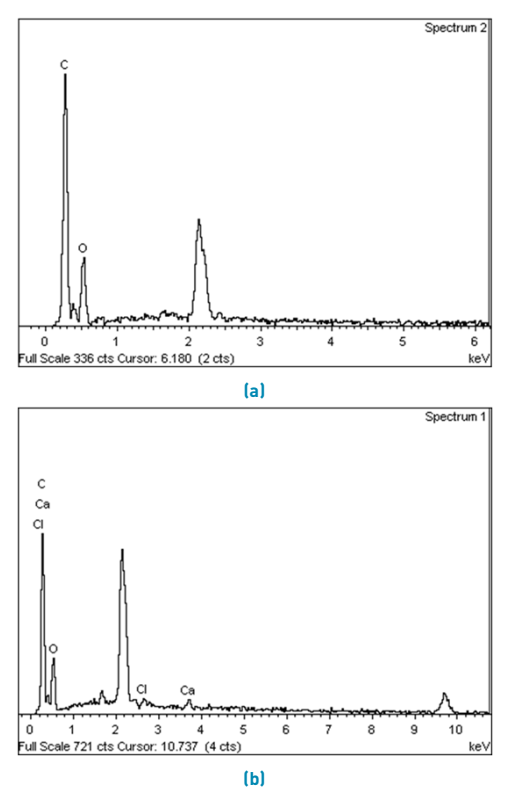
In Figure 2, EDS spectra obtained after dialysis during different timings are shown. Those patterns correspond to the solution dialyzed using (new) dialysis tubing, but represent the behavior of all the other samples. For the solution treated with 7 days of dialysis [Figure 2a), there are peaks corresponding only to carbon and oxygen, which are commonly associated with materials with a protein-like composition [25]. On the other hand, for the solution treated for 5 days [Figure 2b) there are not only peaks of carbon and oxygen, but also traces of calcium and chlorine, showing that 5 days is not enough time for efficient salts removal.
4. Conclusions
In summary, after evaluating three separation methods degummed fibers with sodium carbonate showed several differences within Raman spectra compared with untreated silk fiber, appearing to promote an easy and more effective separation of sericin and fibroin than treatments with distilled water or liquid neutral detergent. Also, food casing showed appropriate results for the purification process in comparison to the new and reused dialysis tubes. This not only reduced the cost of the process but also conferred the added advantage of working with an easily acquirable material. Finally, dialysis requires about five days to significantly remove salts present in the fibroin solution. However as evidenced in the results of EDS, the process should continue until day seven to ensure complete removal of residual salts used in separation and dissolution processes.