1. Introduction
Non-centrifugal cane sugar (NCS) is a solid product sold in different forms (tablets or bricks, cones, round); it is the result of the evaporation of sugarcane juice (Saccharum Oficinarum), without centrifuging, maintaining its constituent elements as sucrose, glucose and minerals [1].
Its name varies depending on the country; Panela (Colombia, Guatemala, Panama, Ecuador and Venezuela), rapadura (Brazil), chancaca (Peru and Bolivia), piloncillo (Mexico), raw sugar, brown sugar, muscovado (USA), jaggery (Africa and Asia), gur (Asia), moscavado and panutsa (Philippines), kokuto and black sugar (Japan) [2]. Sugarcane is the third most important commodity in the world, with a larger production in the Americas (54.1%), followed by Asia (39.1%), Oceania (1.7%) and Africa (5.1%). Colombia ranks seventh among the countries with the highest production in the world with 34.535.753 MT (FAO, 2016). It is preceded by countries such as Brazil, India, China, Thailand, Pakistan and Mexico [3]. NCS is consumed for two main reasons: it is used as food for feeding the population due to its nutritional quality, and second, it is as a sweetening ingredient for other foods [4]. It has a high sucrose content (765.5-894.8 g kg-1), followed by reducing sugars (36.9-105 g kg-1), water (15-158 g kg-1), minerals (3-36 g kg-1), protein (3.7-17 g kg-1) and fats (0-1 g kg-1) [5]. Its consumption has a positive effect on the immune system. It also has anti-toxicity and cytoprotective effects, anticariogenic effects, diabetes and hypertension effects; positive health effects can be due to the presence of minerals and antioxidants [2].
The process to produce NCS begins with the milling of the sugarcane to obtain juice, as the main product, and bagasse as a by-product, which constitutes the fuel for the concentration of the juices. Afterwards, the juice goes through a pre-cleaning system, where solid impurities are removed. Then, it goes to the clarification stage where the suspended solids are removed. After that, the juice goes to the concentration zone in open heat exchangers or "pan" where it is concentrated to 70°Brix. This concentration is obtained through the energy coming from the bagasse obtained in the grinding.
The NCS production requires a higher concentration of soluble solids between 92 to 95. °Brix [6]. Finally, NCS goes through a process of beating, molding, cooling, demolding, packaging and commercialization.
The technological development in NCS making process has focused on ensuring the self-sufficiency of the system, by increasing its energy efficiency. In order to achieve this, AGROSAVIA-CIMPA (Colombian Corporation for Agricultural Research - Research Center for the Improvement of NCS Agroindustry) has worked both in improving the efficiency of the combustion system and heat transfer from the combustion gases towards heat exchangers or pan. In the first case, the combustion chambers have been modified to take advantage of the calorific value of bagasse. Although they have different models, the most efficient to date consists of the "Ward Cimpa" furnace, which allows the pre-drying and complete combustion of the bagasse, by having a second air inlet, which allows reaching a greater temperature of combustion gases. As for the increase of the efficiency of the heat transfer process, different types of pans (finned, fire-tube, hemi-spherical) have been designed, looking for greater contact area and increased heat transfer coefficients.
In this study, the objective was to evaluate the effect of the two technologies (traditional furnace with flat combustion chamber and hemi-spherical pan, and improved furnace with ward combustion chamber and modified pan) on the physicochemical parameters throughout the agroindustrial manufacturing process.
2. Materials and methods
2.1 Plant material
The plant material (cane variety RD 75-11) was cultivated in the fields located in the Department of Boyacá, in central Colombia, in the eastern Andes. Sugarcane was harvested at its maturity stage suitable to produce NCS (16 months).
2.2 Processing technologies
The compositional changes of the sugarcane juice were analyzed in two NCS production technologies: traditional furnace with flat combustion chamber and hemi-spherical open pan (T1), and improved furnace with ward combustion chamber and modified open pan (T2). Both processing technologies were operated with a mixed flow.
The traditional furnace used for the study had five hemi-spherical open pans in stainless steel. The floors and walls that supported the open pan, as well as the chimney were constructed in brick and semi-refractory mortar. The furnace had a flat combustion chamber, in which the thermal energy needed to carry out the production process of NCS was generated. The juice was manually transferred from one pan to another.
The improved furnace had modified pan with greater area of heat transfer, in which fins were used. In total, seven pans were used in the improved furnace, three open pans with fins and four hemi-spherical open pans, made of stainless steel. The furnace had a ward combustion chamber, in which the bagasse was burned. In this type of furnace, the juice is transferred by gravity through a pipe.
2.3 Sample Preparation and processing
The harvested sugarcane was stored for 12 h, and then it was fed to the mill to extract the juice. The bagasse or residue of the cane was taken to the bagasse stocking area where it was subjected to a natural drying process. The juice entered the making process where it was cleaned, concentrated, beaten and molded to obtain the NCS.
2.4 Sampling
Sampling was started from the milling of the cane. Each batch lasted approximately 3 hours. Three lots were sampled for each NCS processing plant. Sampling was performed in 9 points: X1 = mill (22 °C), X2 = pre-cleaner 1 (22 °C), X3 = pre-cleaner 2 (22 °C), X4 = clarifier 1 (95 °C), X5 = clarifier 2 (96-97 °C), X6 = syrup (105 °C), X7 = NCS point (120 °C), X8 = after whipping (100 °C) and X9 = NCS (22 °C). At each sampling point and in each lot, 3 samples of 1 Liter were taken every hour, except at the sampling points; X7, X8 (300 mL) and X9 (250 g of NCS). All samples were frozen at -30 °C until analyzed. Samples X6, X7, X8 were taken directly into glass containers previously sterilized (121 °C for 15 min), and sample X9 in sterile bags. All physicochemical analyzes referenced below were performed in triplicate.
2.5 Analysis of physical and chemical properties
PH, soluble solid content, total acidity and moisture content
The analytical parameters hydrogen potential (pH) (AOAC 981.12) (S20 SevenEasy pH, Mettler Toledo, Bogotá, Colombia), solid soluble content (SSC) (AOAC 932.14) (digital refractometer, Atago Company Ltd, Tokyo, Japan) and total acidity (TA) (titration with 0.1 N sodium hydroxide solution, Auros química, Bogotá, Colombia) were evaluated at 20°C as described by [7]. For liquid samples (X1, X2, X3, X4, X5, X6), 10 mL was taken directly for pH determination and three drops for solid soluble content determination. For the solid samples (X7, X8 and X9) the same amount of sample was taken but from a dilution of the sample in distilled water 1:10. Previously, the calibration of the pH meter was carried out with reference solutions of pH 4.00, 7.00 and 10.00 (Thermo scientific, Bogotá, Colombia). The moisture content was determined at 105 °C with a moisture scale (AOAC 925.10) (Mettler Toledo HB43-S Halogen Classic Plus, Ohio, USA). All analysis was performed in the Research Center for Engineering Processes in the Jorge Tadeo Lozano University, Bogotá.
Color evaluation
Color at three equidistant points was determined using a chromameter (CR-300, Minolta, Ramsey, NY, USA). Lightness (L*) was determined in a range from 0 (black) to 100 (white). The color component a* varies from green (negative values) to red (positive), the color component b* varies from blue (negative values) to yellow (positive). A BaSO4 plate was used as a reference standard (L = 96.9, a* = 0, b* =7.2).
The results are expressed as color index (CI = [(𝑎∗)(1000)]/[(𝐿∗)(𝑏∗)]), Chroma (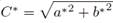 ) and color difference (∆E = [(∆L)2 + (∆a
*)2 + (∆b
*)2]1/2).
) and color difference (∆E = [(∆L)2 + (∆a
*)2 + (∆b
*)2]1/2).
Minerals
0.3 g of each sample was digested in 9 mL of nitric acid: perchloric (5:2) in a microwave digester (Milestone, Ethos Plus, Shelton, US). The digestion program consisted of a ramp time of 5 min to reach 180°C and digestion for 10 min [8]. Phosphorous (P), potassium (K), calcium (Ca), magnesium (Mg), sodium (Na), sulfur (S), iron (Fe), copper (Cu), manganese (Mn), zinc (Zn), arsenic (As) and lead (Pb) were determined using an atomic absorption spectrum (Agilent Technologies 280FS AA, Bayan Lepas, Malaysia). Nitrogen (N) was determined through the EPA method (Environmental Protection Agency) [9] and Boron (B) through the NTC (Colombian Technical Standard) [10].
Reducing sugars
The reducing sugars were determined through the reducing sugars method of Lane and Eynon [11]. The samples were subjected to strong acid hydrolysis to unfold the starches and to obtain glucose.
2.6 Statistical analysis
The differences between the technologies throughout the production process were evaluated using an analysis of variance (ANOVA, P≤0.05; Statgraphics Centurion 18, software shop, Bogotá, Colombia). The differences were identified using a multiple range least significant difference (LSD) test of mean values.
3. Results and discussion
3.1 Effect of the technologies on the change of the pH, acidity and ash during the process of NCS elaboration
The pH of cane juice ranged from 5.18 (T2) to 5.24 (T1), data similar to those reported in Hoya del Río Suárez (HRS), departments of Santander and Boyacá-Colombia (5.36) and Candelaria municipality, Valle del Cauca- Colombia (5.21). The average pH of the zones where sugarcane is cultivated varies between 5.0 and 5.2 [14]. In X4, a pH increase of 18.96% (T1) and 2.66% (T2) was observed, reaching 5.96 pH (T1) and 5.02 pH (T2). This was due to the fact that food grade lime was added in the process to minimize the inversion of the sucrose molecule. The pH should be increased to 5.6-6.0 (molded NCS) and up to 6.4 (granulated NCS) [14]; therefore the pH (T2) was not within these parameters, this may be due to the fact that the initial pH of the juice in T2 was significantly lower than in T1 (p ≤0.05), in addition this is a manual process in which the operator always adds the same amount of lime, without taking into account the measurement of this parameter. Food grade lime acts as a clarification aid, allowing the efficiency in the clarification process to be increased by the destabilization that the pH change produces on the color precursor material, allowing an easy removal and rinsing of the product, besides it creates a protective barrier of the sucrose molecule, impeding its hydrolysis and loss of quality [14]. Significant differences (p ≤0.05) in the interaction between technologies and stages were observed throughout the NCS elaboration process [Figure 1].
Total acidity (TA) in cane juice ranged from 1.39 (T2) to 1.45 (T1) g citric acid L-1 [Figure 2] and no significant differences were found between T1 and T2 (p ≤0.05). However, in X3 there were differences due to the equipment used (pre-cleaner 2) in each NCS processing plant, as well as the amount of impurities of the cane used. In this stage, it is allowed a greater elimination of solids which can affect the content of TA. The raw juice contains impurities such as soil, bagasse and its residues, therefore it is necessary to subject it to pre-cleaning systems, which retain by physical means the greatest amount of impurities [14]. However, both the pre-cleaner design and the operation, since it is manual, can affect the removal of impurities. In X4 with respect to X3, a decrease of 72.82% (T1) and 64.28% (T2) occurred possibly due to the addition of a mucilage called balso (Heliocarpus americanus L), which is bark of a tree previously macerated and filtered and acts efficiently agglutinating the particles and allowing their removal. Its binding action results from the clarifying action with juices and heat, forming dark, dense and consistent flocs called "cachaza", which are removed before the boiling process [14].
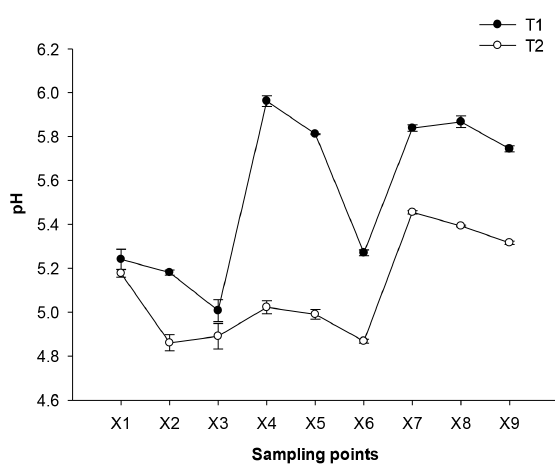
Figure 1 Effect of the technologies on the change of the pH of NCS elaboration. Mean (n = 3) ± SE. Analysis of variance (p ≤ 0.05) showed as significant factors the sampling points and processing technologies. LSDsampling points = 0.034, LSDsampling points-processing technologies = 0.048
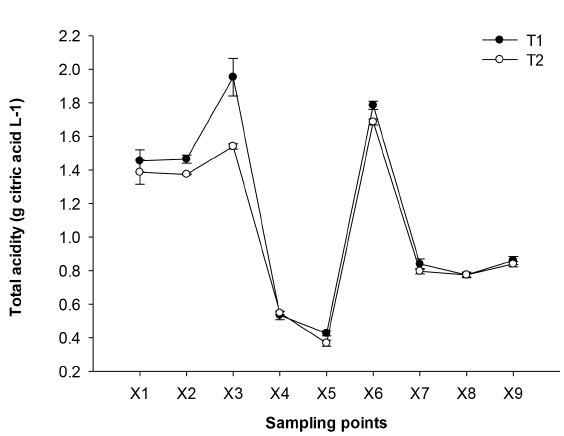
Figure 2 Effect of the technologies on the change of the acidity (g citric acid L-1) of NCS elaboration. Mean (n = 3) ± SE. Analysis of variance (p ≤ 0.05) showed as significant factors the sampling points and processing technologies. LSDsampling points = 0.087, LSDsampling points-processing technologies = 0.123
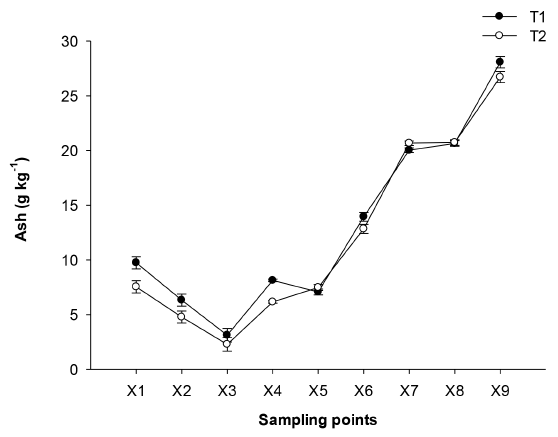
The values of ash depend on the composition of the cane and the juice, they presented a similar behavior during the process [Figure 3]. A decrease was observed in the early stages X1, X2, and X3, due to the large amount of solids (cane particles, bagasse, impurities) that are removed. From X4, an increase in the ash content was observed because the concentration of the cane juice begins. In X9, the NCS was obtained with 26 g kg-1 of ash, a value that is between the range reported (3-36 g kg-1) [5], (11.5-26.3 g kg -1) [15] and the existing regulations (minimum 8 g kg-1 for NCS blocks and minimum 10 g kg-1 for NCS granulated) [1]. In X4 and X5 not all the salts were removed from the juice, but they remained in it until obtaining the NCS, which could affect its quality. Ash in sugarcane juice is an important aspect that refers to the soluble inorganic salts contained in sugarcane juice. [16] mentioned that a high ash/reducing sugar ratio would increase the likelihood of loss of sucrose for molasses and would effectively affect the yield and quality of the raw sugar.
3.2 Effect of technologies on solid soluble content (SSC)
The SSC ranged from 17.51 to 18.86 °Brix and no significant differences were found between T1 and T2 [Figure 4], these values were lower than those reported [17] with 19.99 °Brix, HRS-Colombia (21.65 °Brix), Candelaria- Colombia (20.4 °Brix) and Cuba (19.29 °Brix). However, the results can fluctuate between 16-24 °Brix but ideally they should be as high as possible. The SSC variability depends on the agronomic conditions and climatic conditions, such as age, rain cycles, light hours and maturity of the cane [14]. In the first stages (X1 to X4), the SSC remained constant since these are stages of cleaning and clarification, then from X5 to X9 a progressive increase was observed. In X6, a significant difference (p ≤0.05) was observed between the two technologies with respect to X5, where T2 concentrated the sample more (3.24 vs 3.03 times) than T1, because the improved furnace design with ward combustion chamber and modified pan (T2) can increase efficiency by the temperature reached in the chamber (1200 °C) as opposed to T1 (900 °C). At X7 and X8, there were no significant differences (p ≤0.05) between the technologies concentrating the SSC 1.2 times and 1.01 times, respectively. Finally, SSC at X9 ranged from 89.88 °Brix (T1) to 90.32 °Brix (T2) and no significant differences (p ≤0.05) were found between T1 and T2 (p ≤0.05). Similar values to those reported (90-96 °Brix) [14] but lower than those reported [17] for the same variety RD 75-11 with 98.1 °Brix. Through evaporation, it was possible to pass on average 18.19 °Brix in clarified juices to 67.08 °Brix syrups and to NCS of 90.1 °Brix, with a concentration of juice to NCS of 5.19 times (T1) and 4.86 times (T2).
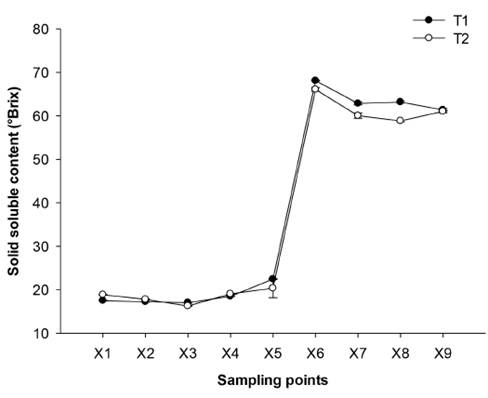
Figure 4 Effect of the technologies on the change of the solid soluble content (°Brix) of NCS elaboration. Mean (n = 3) ± SE. Analysis of variance (p ≤ 0.05) showed as significant factors the sampling points and processing technologies. LSDsampling points = 0.528, LSDsampling points-processing technologies = 1.585
3.3 Effect of technologies on color
In X1, significant differences (p ≤0.05) were observed in cane juice with Chroma values of 6.25 (T1) and 12.34 (T2), indicating that the color of T2 was more saturated than T1, both in cane juice and in the product (X9), also ΔE* indicated that the color difference is greater in X9, with significant differences (p ≤0.05) between T1 and T2, starting from the same cane variety [Table 1]. NCS can have different colors depending on the raw material used, the variety of the cane, the agro-ecological conditions and the elaboration process [1].
Table 1 Effect of technologies on color
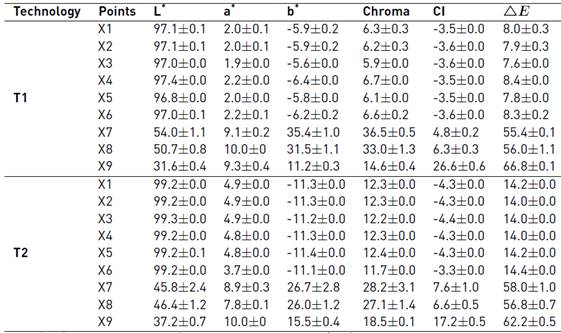
Mean (n = 3) ± SE. Analysis of variance (LSD’s multiple range tests, LSD p ≤ 0.05) showed significant differences in the parameters of chroma (LSD = 1.289), CI (LSD = 0.723) and ΔE (LSD = 0.722).
In the last stages (X6, X7, X8, X9) a significant decrease (p ≤0.05) in the L* values was observed, whereas the values of a*, b*, C*, IC, ΔE* increased, a similar behavior to that reported [18]. The values of L* decreased by the evaporation of the juice, solids concentration and caramelization of the sugars, the values of a* turned to red and the values of b* to yellow. The CI changed from a yellow color in X1 (-3.54 T1, -4.33 T2, CI <0 = yellow), then an intense yellow color in X8 (6.28 T1, 6.57 T2, CI <5 = intense yellow), to a color in the final product (X9) from deep orange to deep red (26.56 T1, CI +20 to +40) and from pale yellow to intense orange (17.24 T2, CI +2 to +20). This change of color is due to the fact that in X6 about 90% of the water evaporates and it is transformed into a sweet, dense and viscous liquid of variable color. X7 is a critical stage of the process at high temperatures (100-125 ° C) in which antifoaming agents are added to prevent syrup from adhering to the walls, caramelizing and burning [14], which would directly affect the color. Finally, in X8 and X9 the color and consistency is determined directly by SSC and temperature; If it is removed at a high temperature, it will present a caramelization of the sugars with its consequent darkening, otherwise its solidification will be difficult. NCS without chemical additives presents a progressive darkening by the action of the light [14]. According to the norm, it is the use of dyes, sodium hydrosulphite or sodium hyposulphite, or other chemical substances with whitening properties is not allowed [1].
3.4 Composition of minerals in syrup and NCS
The major minerals in X6 and X9 were K, Ca, N, Mn, Fe, B, Cu and Zn [Table 2]. Both K and Ca decreased in X9 when using T2, by 47.7% and 22.73% respectively, this can be attributed to the temperature of the combustion chamber in T2.
Table 2 Composition of minerals in syrup and NCS

Mean (n = 3) ± SE. Values in rows followed by the same letter are not significantly different by LSD (p ≤ 0.05) according to LSD’s multiple range test, ns=not significant. Analysis of variance showed significant differences in the parameters of N (LSD = 0.022), P (LSD = 0.028), K (LSD = 0.065), Ca (LSD = 0.021), Na (LSD = 0.005), S (LSD = 0.008), Fe (LSD = 2.001), Mn (LSD = 1.570), Zn (LSD = 0.538), As (LSD = 0.551) and Pb (LSD = 0.176)
The contents of Mn, Fe, Cu and Zn increase from X6 to X9, due to the concentration of the samples. The values of K in NCS (4.7 g kg-1 T1, 2.40 g kg-1 T2), Ca (2.8 g kg-1 T1, 1.7 g kg-1T2) and P (0.2 g kg-1 T1; 0.4 g kg-1 T2) were higher than that required in the standard (1 g K kg-1; 0.1 g Ca kg-1; 0.05 g P kg-1)1. However, the Fe values in NCS (0.009 g kg-1T1, 0.012 g kg-1T2) are below those required in the standard (0.015 g kg-1) [1]. Sugarcane contains major elements (N, P, K), intermediate nutrients (Ca, Mg) and secondary nutrients (S, Fe); Fe is necessary in the synthesis of chlorophyll and is an essential component for the enzymatic activity [14]; however, according to the results obtained, it is possible that the agronomic conditions and the variety did not allow a higher Fe content in the final product. Other authors also reported higher K and Ca content in NCS (5.31 g K kg-1, 5.0 g K kg-1 and 1.02 g Ca kg-1, 2.5 g Ca kg-1) [5, 19]. NCS is a natural sweetener and provides a higher mineral content than syrup (0.96 g K kg-1 and 0.26 g Ca kg -1) [19] and refined sugar powder which does not have a mineral content [20]. K is found in fruits and fresh vegetables, whole grains, meats, salmon, milk, yogurt and pumpkin, is necessary for proper balance of fluids, the nervous and muscular system, proper maintenance of blood pressure and waste disposal [21].
The K values obtained in NCS (4.70 g kg-1T1; 2.40 g kg-1T2) are similar to those found in bananas (3.58 g kg -1), broccoli (3.16 g kg-1), and higher than those found in products such as grape (2.03 g kg -1), grapefruit (1.48 g kg-1) and watermelon (1.12 g kg-1) [20]. Ca is found in broccoli, mustard leaves, milk, fortified tofu, fortified soy milk, salmon and sardines. It is important for healthy bones and teeth, muscle relaxation, the nervous system, the immune system, the circulatory system and the regulation of blood pressure [21].
The Ca values obtained in NCS (2.80 g kg-1T1; 1.70 g kg-1T2) are higher than those found in broccoli (1.20 g kg-1), mustard leaves (1.15 g kg-1), milk soybeans with added calcium (1.24 g kg-1) and lower than those reported for tofu (3.72 g kg-1).[21] Mn is a micromineral available in hazelnuts, squash, beans, spinach, brown rice, mussels, fish, whole wheat and tofu. It is important for the normal functioning of the brain and the nervous system; it is also useful for women during the postmenopause and for the prevention of osteoporosis [21]. The Mn values obtained in NCS (0.012 g kg-1) are similar to those found in pistachio (0.01 g kg-1) [20]. These results suggest that NCS is a potential source of important minerals in the prevention of various diseases.
3.5 Reducing sugars
Reducing sugars X1 (16.9 g kg-1) did not present significant differences (p ≤0.05) in T1 and T2 (data not shown). These values are within the range (10-18 g kg-1) reported [17] considered normal for the NCS crystallization process. In X9, the reducing sugars presented a significant difference (p ≤0.05) being higher in T2 (70.8 g kg-1) than in T1 (66.6 g kg-1). However, in both cases the normative is met (minimum 55 g kg-1) [1]. According to the results obtained, it is possible that at T2 (> temperature combustion) a higher acid hydrolysis of the polysaccharides occurs during cooking, which releases other simpler sugars [22]. In time, syrup with a content greater than 70 °Brix tend to be separated into two phases: one semi-solid (sucrose) and another one super-rich in reducing sugars (glucose and fructose). Glucose is one of the monosaccharides present in cane juice, is the most stable form, it crystallizes as a monohydrate, and forms rhomboid crystals having a melting point of 146 °C (anhydrous) and 83 °C (hydrated). Fructose is more soluble in water than glucose and melts at 102-104 °C [14].
3.6 Heat capacity
The energy values obtained [Table 3] are similar to those reported for NCS (15700 J g-1, 15533 J g-1) [20, 23] higher than those reported for sugar beet (12727 J g-1) [20], syrup (13062 J g-1) [23], and lower than those reported for refined sugar (16747 J g-1) [20]. Current consumer trends are framed towards the consumption of products free of preservatives, colorants and additives; beverage companies grow exponentially and, being aware of these changes, they increasingly cover a wide range of products with new features, flavors and forms. For this reason, NCS could be included in drinks as a natural sweetener and a source of minerals in the diet.
Table 3 Heat capacity in NCS
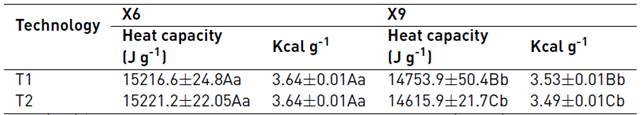
Mean (n = 3) ± SE. A-B: Within a row, different letters indicate significant differences (p < 0.05). a-b: Within a column, different letters indicate significant differences (p < 0.05) according to LSD’s multiple range test. Analysis of variance showed significant differences in the parameters of heat capacity (LSD=0.044).
3.7 Moisture content
There were significant differences (p ≤0.05) in T1 technology (52.2±2.14 g kg-1 X7; 56±1.5 g kg-1 X8; 59.5±1.17 g kg-1 X9) with respect to T2 (72.7±0.7 g kg-1 X7; 73.5 ±1.2 g kg-1 X8; 75.6±1.1 g kg-1 X9) (data not shown). However, with both technologies the moisture content of NCS was as required in the standard (Maximum 90 g kg-1) [1]. The moisture content is of great importance to avoid the deterioration of the NCS due to the development of microorganisms on the surface, like molds and yeasts (Maximum 150 UFC g-1) [1].
4. Conclusions
The NCS making process started with cane juice with average values of 5.21 pH, 1.42 g citric acid L-1, 8.6 g L-1 ash, 18.19 °Brix and 16.9 g L-1 of reducing sugars, obtaining NCS with average values of 5.53 pH, 0.85 g citric acid L-1, 26 g L-1 ash, 90.1 °Brix and 68.7 g L-1 reducing sugars.
The cane juice presented significant differences (p ≤0.05) in color (Chroma of 6.25 T1 and 12.34 T2) even within the same variety (RD 75-11). During the process, a change in color was observed in the cane juice (CI <0 = yellow), in the evaporation (CI <5 = intense yellow) and finally in the NCS (CI +20 to + 40 = intense orange to deep red, CI +2 to + 20 = pale yellow to intense orange).
The technology with improved furnace with Ward combustion chamber and modified pan (T2) managed to concentrate the CSS of syrup (X6) to NCS (X9) in a 36.03 (%) unlike T1 (32.59%). Additionally, a higher texture in the finished product and a higher concentration of reducing sugars (70.8 g L-1) was obtained as opposed to T1 (66.6 g L-1). However, with this technology K was reduced by 47.77% and Ca by 22.73%.
NCS has a high calorific value (14684.9 J g-1) and could be used as a natural sweetener in energy drinks. In addition, it is a source of minerals and has properties which are beneficial to health.