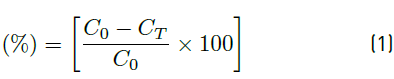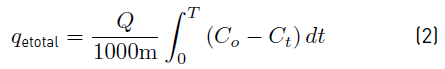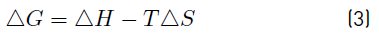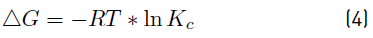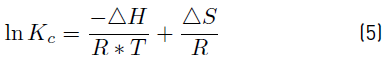1. Introduction
Heavy metals are continuously released from different sources such as industrial, agroindustrial and domestic waste into the environment, generating problems for human and aquatic organisms. The contamination of water resources, due to the indiscriminate use of heavy metals such as cadmium, zinc, cobalt, nickel, chromium, among others, has been a worldwide concern in recent years, as a result of accelerated industrialization and urbanization [1, 2]. Chromium is one of the most toxic heavy metals. Its wide distribution in the earth's crust and its multiple applications in industries that include mining, electrodeposition, leather, and pigment production means that it can be found in the wastewater [3, 4]. If ingested beyond the maximum concentration (0.1 ppm), it can cause health disorders, such as vomiting and haemorrhage [5, 6].
Due to the adverse effects of Cr (VI) on human health and aquatic ecosystems, several chemical and physical technologies have been developed to remove metal ions from wastewater, including ionic exchange, solvent extraction, chemical precipitation, membrane filtration, and electrochemical treatment [7]. However, these traditional techniques have their inherent limitations, such as low removal efficiency at low concentrations, sensitive operating conditions and high operating cost. On the other hand, the Colombian regulation about the punctual discharge to bodies of marine water establishes that the Cr (VI) discharge concentrations must be between 0.2-0.5 ppm, under Resolution 0883 of May 2018 [8]. However, concentrations of this metal could reach up to 450 ppm, for industrial effluents [9, 10]. Among the various physicochemical processes, adsorption is one of the most effective and easy to implement methods [11, 12].
Currently, the use of agricultural waste as an adsorbent is being widely considered due to its accessibility, low cost and its physicochemical characteristics, also, these agricultural waste materials could take an important role in the national economy when being used correctly [13]. Plantain is one of the main fruits that are consumed worldwide in large quantities. Despite its different uses, a large amount of plantain peels is wasted every year, and its elimination states a serious problem. The application of plantain peels as an adsorbent is advantageous for the elimination of the various water pollutants, since being a material of lignocellulosic nature; it is related to functional groups such as hydroxyls, carboxyls and amines, which have an excellent capacity for joining metal ions using a pair of electrons to form complexes in solution [14]. Thus, for the development of the present work, the efficiency of the biomass in the continuous system was evaluated by varying the temperature, bed height and particle size. This study was done to determine the optimum condition in which a higher percentage of chromium concentration is removed. Moreover, the effect on the thermodynamics of the process was analysed.
2. Materials and methods
2.1 Experimental design
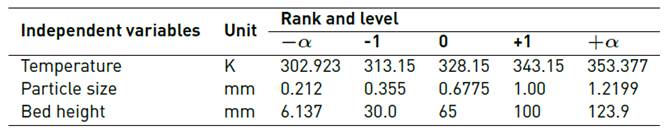
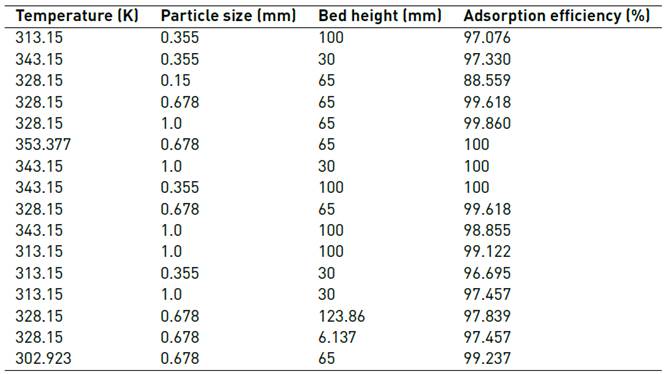
A central composite design was carried out to study the adsorption tests in a continuous system: 22 + rotatable star with 3 levels of variation (temperature, particle size and bed height). The STATGRAPHICS Centurion XVII software was used, as shown in Table 1.
2.2 Preparation of the adsorbent
The plantain peels were supplied by local businesses in the city of Cartagena (Bolívar) as a residue of their food production processes. The preparation of the material consisted of a wash with water to remove the dirt. Then, a manual size reduction was carried out to facilitate its handling in the later stages, a wash with distilled water was carried out to eliminate tannins, resins or other compounds that can affect the biosorption process [15]. Finally, it was dried in the oven for 8 h and 279.15 K. This material was milled and sieved in different sizes, and the size between 0.15 and 1 mm was chosen. The chemistry of the surface of the material was evaluated through FTIR spectroscopy before and after the adsorption process to determine the functional groups involved.
2.3 Adsorption tests in continuous system and effect study
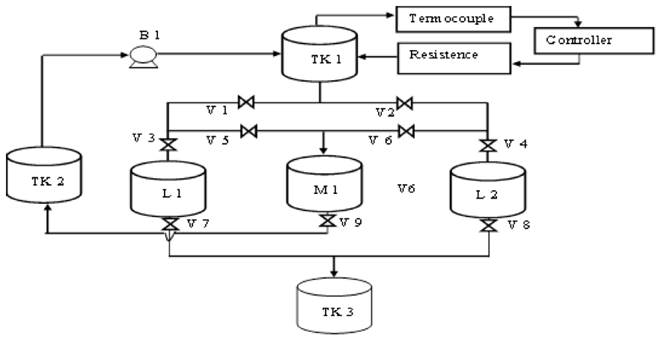
The removal capacity of the biomaterial was evaluated by performing tests on the packed bed equipment with continuous flow in an acrylic column as is shown in Figure 1.
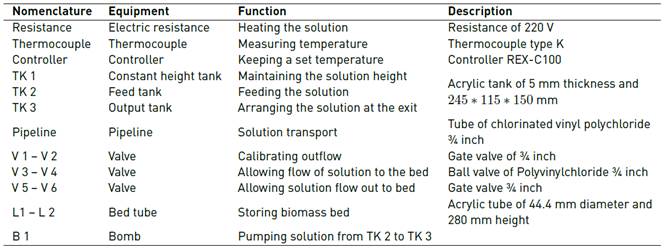
The description of the elements of the continuous adsorption equipment is shown in Table 2:
The solution containing the ions under study flowed to the column by gravity, with a flow rate of 0.75 mL/s, being adjusted using a globe valve. The column was packed with the biomass by varying the temperature, particle size and bed height, in order to determine the optimal condition and the influence of each parameter. The variation and control of the temperature were carried out using a REX C-100 temperature controller. The Cr (VI) solution at 100 ppm was prepared from 283 mg of potassium dichromate (K2Cr2O7) per litre of water.
Samples to measure the final concentration of the ions in the solution were taken at the lower outlet of the column. The concentration data were determined with UV-VIS spectrophotometry using the colourimetric method of 1.6 Diphenylcarbazide at 540 nm [16]. The removal efficiency (%) for each condition was determined based on Equation (1) [17]:
Where C T is the remaining concentration of the ion (mg/L) and C 0 the initial concentration in the solution, indicates the value of the actual removal percentage by the continuous adsorption system. The statistical analysis of the experiments was carried out using an ANOVA analysis of variance.
2.4 Break curve
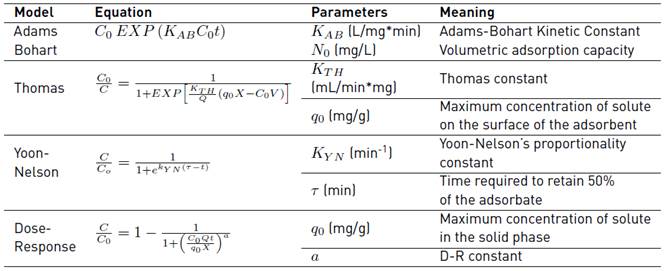
The rupture curve is performed to establish the shelf life and saturation point of the bed as the solution circulates over time. Thus, the Cr (VI) adsorption advance curve using plantain peel as a filling packed of the continuous bed was used at the best experimental conditions found after the execution of the tests proposed in the experimental design of Table 1, during 480 min, for an initial concentration of 100 ppm. Then, the adjustment of the data to the adsorption models in the continuous system was performed using the OriginPro 8 software, and they are shown in Table 3 [18, 19].
Subsequently, the Equation (2) was used to calculate the capacity of the column (mg/g):
Where, Q is the flow rate (mL/min); m, the amount of adsorbent (g); C 0 and C t , the initial and final concentration of the metal in solution (mg /L) [20].
2.5 Thermodynamics of the process
In order to calculate the thermodynamic parameters, the graphical method based on the Van't Hoff equation was used, therefore, the change in standard Gibbs free energy (ΔG) was estimated by using Equations (3) and (4), enthalpy (ΔH) and standard entropy (ΔS) using Equation (5) in its graphic form.
Where Kc = Cac/ Cse; Cac is the concentration of the adsorbate at equilibrium contained in the surface of the adsorbent; Cse is the solution concentration at equilibrium and R is the universal gas constant (8.314 J/mol·K) [21]. The (ΔH °) and (ΔS °) are determined from the slope and the intercept with the axis and the Arrhenius graph of lnKc vs T -1, respectively, by applying the Van’t Hoff graphical method [22].
3. Results and discussion
3.1 Characterization
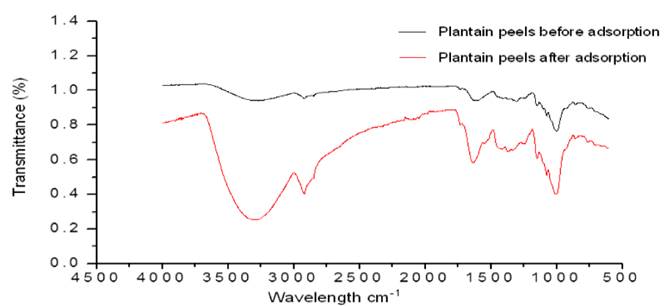
From the FTIR analysis shown in Figure 2, functional groups contained in the biomaterial and those that can participate in the process were determined.
From the process before and after the adsorption, the change in the intensity of peaks in the wavelengths of 3,300 cm-1 corresponding to the -OH functional group is observed, at 2,914 cm-1 it is related to the vibrations of the CH bonds, at 1748 cm-1 with the group -NH2 and at 900 cm-1 were attributed to the vibration of the CM (carbon-metal) bonds. Thus, the OH and NH2 groups are related to the bands responsible for the formation of complexes with the cations in solution, and the CH group does not participate directly in the formation of the complex, however, its decrease can be attributed to an equalization of the intensity due to the formation of complexes [23].
3.2 Adsorption tests and effects study
In Table 4, the adsorption efficiency of the different conditions studied is shown, finding that the proposed biomaterial present favourable adsorption results. This fact may be associated with the lignocellulosic characteristics of the material related to the presence of hydroxyl, carboxyl and amino functional groups that have been related to the high metal removal [24].
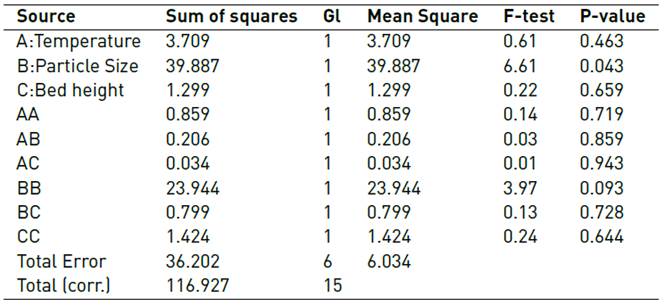
Table 5, ANOVA divides the variability of adsorption efficiency into separate pieces for each of the effects. Then, it tests the statistical significance of each effect by comparing its mean square against an estimate of the experimental error. In this case, the particle size has a P-value of less than 0.05 indicating that it is significantly different from zero with a confidence level of 95.0%, being the only significant effect on the adsorption of Cr (VI).
The R-Square statistic indicates that the model, thus adjusted, explains 69.039% of the variability in the adsorption efficiency. The adjusted R-squared statistic, which is more suitable for comparing models with a different number of independent variables, is 22.597%. The standard error of the estimate shows that the standard deviation of the residuals is 2.456. The absolute mean error (AME) of 1.138 is the average value of the residuals. The Durbin-Watson (DW) statistic tests the residuals to determine if there is any significant correlation based on the order in which the data is presented in the file. Since the P-value is higher than 5.0%, there is no indication of serial autocorrelation in the residuals with a level of significance of 5.0%.
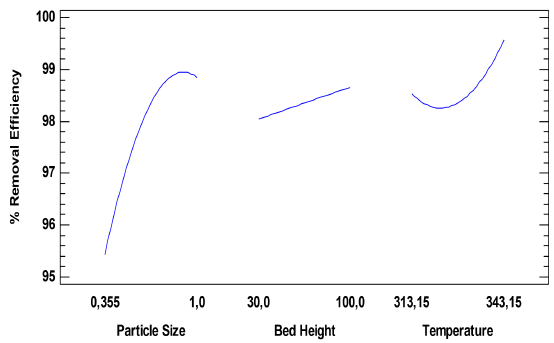
In Figure 3, the effect of the parameters studied on adsorption efficiency is plotted, thus it is established that as the particle size, which is the only significant one, increases, so does the removal efficiency, which may be related to the decrease of the stagnation of the solution, which facilitates and improves the transit of fluid through the bed.
However, at particle sizes of 1 mm, a decrease occurs as a result of a slower pore diffusion rate because the diffusion trajectory along the pores is high as well as the resistance to diffusion [25, 26]. In the case of temperature, the increase in removal efficiency due to the increase of this parameter could be due to the binding between the adsorbate and adsorbent by the formation of new active sites. These sites could form new bonds between the ions and the active functional groups on the adsorbent, overcoming the barrier of activation energy and improving the rate of intraparticular diffusion [27]. For the bed height, the behaviour could be explained by the increase in the penetration time of the solution, which represents a longer residential solution time in the bed column. Consequently, the metal has more time to diffuse into the solid phase. This fact proves that at the highest level of the bed, the diffusion towards the solid phase dominates over the axial dispersion although it is observed that at given bed height, a maximum of ion retention is reached by the surface of the material, which affects the decrease in removal at such a bed height [28].
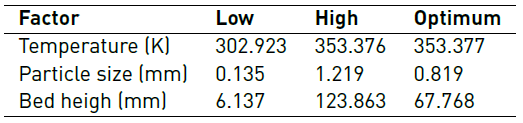
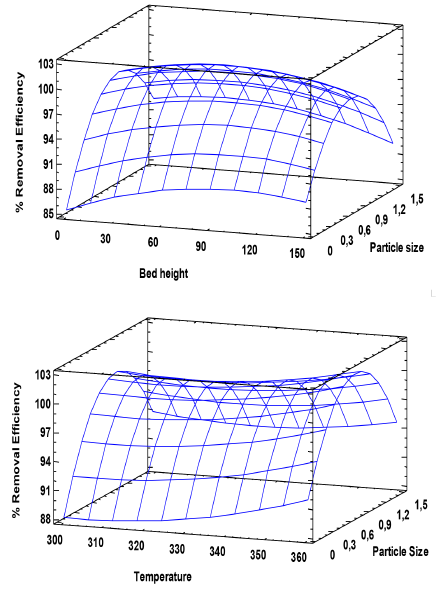
Table 6 shows the combination of the factor levels, which maximizes the removal efficiency over the region indicated in Figure 4.
In practice, the smaller pore sizes produce stagnation of the solution in the upper part of the bed so that the outflow of the liquid in the lower part decreases. Due to this, a mean bed height with an average particle size ensures proper execution of the experiments. Regarding the temperature, the highest one was taken as optimum, which suggests a possible endothermic process, favoured with an increase in temperature. However, it is noted that the other temperature values evaluated also show good percentages of removal, between 88 and 99%, considering that in practice, additional energy consumption, referred to the heating of the solution, would not be necessary.
3.3 Rupture curve modeling
Factor values that resulted in a higher removal were selected and were implemented for the configuration of the packed column and operation of adsorption tests. To establish the useful life and breakage of the bed through an advance curve during a period of 480 min, where this better condition was corroborated due to the high removal made by the adsorption column for an initial concentration of 100 ppm Cr (VI), pH 2, 303.15 K, and flow rate of 0.75 mL/s.
It is observed that the breaking time of the column is 300 min, where the output concentration was 7.3 ppm, and therefore, the removal percentage was 92.7%. Then, using Equation (2) and calculating the area under the curve, it was determined that the total amount of metal removed after 8 h of experimentation is 989.191 mg corresponding to 86,856% of total removal. Likewise, as the solution continued to flow, the output concentration began to increase. However, during the 8 h of the process, the saturation of the material was not reached, indicating the high adsorption potential of the plantain peel [20].
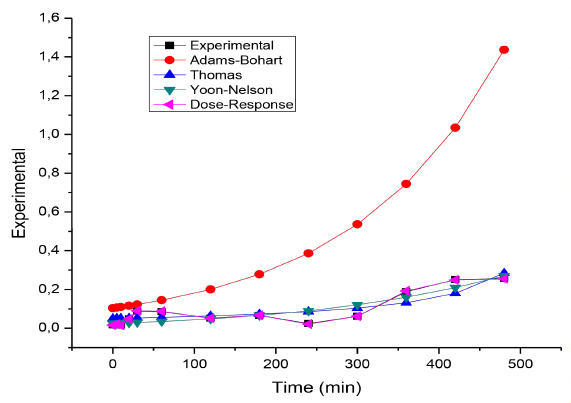
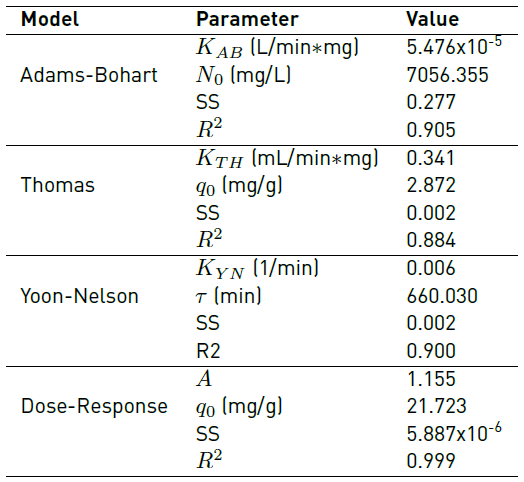
Table 7 shows the adjustment of the Response Dose, Yoon-Nelson, and Thomas models to the experimental data and the calculated parameters.
The best-fitted model in the column adsorption system was chosen according to the determined correlation coefficient (R2) [29]. From the adjustment of Figure 5 and the values of the sum of errors (SS) and R2, it is established that the process behaviour is described by the Dose-Response model, which adjusts to the entire advance curve. Also, it is observed that the q 0 calculated by the model (21.723 mg/g) is very close to the experimental value obtained (25 mg/g). The adjustment of the entire rupture curve to this model was also reported in similar works with olive seeds [30], Spirulina platensis cyanobacteria [31], orthophosphoric acid-activated lignin [32], Chinese pumpkin [33], cocoa shell [34, 35], allspice [36], among other biomaterials used for the removal of Cr (VI), reaching adjustments higher than 92%. In this way, the adjustment to the Dose-Response model allows, through the value of its parameters, to obtain an expression that reproduces the behavior of the columns in other experimental conditions, without the need for additional experiments, maintaining the same operating conditions of the system fixed bed as well as its scaling [35].
3.4 Thermodynamic parameters
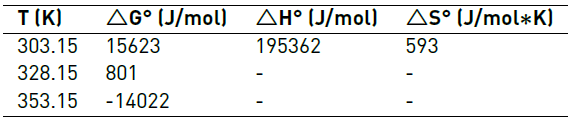
The results of the thermodynamic parameters calculated for the adsorption of Cr (VI) plantain peel are shown in Table 8.
The positive values in the change of enthalpy (ΔH°) indicating that the adsorption process is endothermic, as the diffusion is an endothermic process, the temperature of the solution could have increased the adsorption of ions from of the aqueous solution [37]; this is evidenced in the adsorption results presented in Tables 6 and 8. In addition, the value of the enthalpy change (ΔH°) is 195362 J/mol, which is in the range between 20000 and 400000 J/mol, thus, it is suggested that the process is dominated by a chemical adsorption mechanism, where the functional groups on the surface of the adsorbent behave as active sites that can interact through chemical bonds [38, 39]. On the other hand, the positive value in the change of entropy (ΔS°) warns the possible reversibility of the process, indicating an increase in the randomness in the liquid-solid interface during the adsorption process, which suggested that the ions of Cr (VI) could replace some water molecules previously adsorbed on the surface of the adsorbent [40, 41].
On the other hand, the change in the Gibbs free energy (ΔG°) decreased proportionally with the increase in temperature, indicating that the process of adsorption of Cr (VI) in aqueous solution becomes more favorable at higher a temperature, which provides an increase in the removal capacity of the studied ions. However the system presents two stages in the adsorption process, the positive values of the change of the Gibbs free energy (ΔG°) indicate a non-spontaneous system for adsorption, since it is unable to evolve by itself, meanwhile the negative value suggested that the process of adsorption of Cr (VI) with the plantain peel is thermodynamically feasible at a temperature of 353.15 K [42].
4. Conclusions
The removal of Cr (VI) in a continuous system using plantain peel was efficient to the studied conditions, determining that, from all the evaluated parameters, only the particle size is significant in the adsorption efficiency. A temperature of 353.15 K, the particle size of 0.82 mm and a bed height of 67.768 mm was found as an optimum condition. According to the thermodynamics of the process, chemical adsorption is suggested, establishing that the predominant mechanism may be the formation of complexes between the metal and the functional groups of the adsorbent, it is also determined that the process is endothermic, and it is favoured at high temperatures. Although the process has an endothermic nature, at temperatures evaluated in the design of experiments, good removal percentages are obtained. The results obtained in the present investigation suggest that the Dose-Response model can be used reliably to scale the process, considering the variables involved.