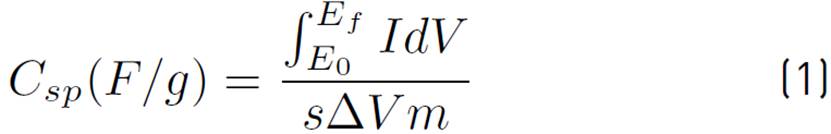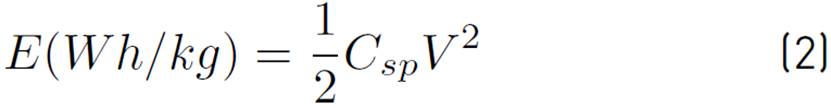1. Introduction
Modern life requires a huge amount of energy. One of the most demanded sources is the energy stored in electrical devices; telecommunication devices, energy reservation systems, and electric vehicles, among others, are some of the top applications requiring stored energy. The devices used for energy storage applications must have high power and energy densities; they must be made of low-cost and long-lasting materials[1]. The present energy demand has resulted in the search and development of new energy storage devices, which must be more efficient, portable, and high-power deliverers. Supercapacitors are devices with a higher energy density than common capacitors and a higher power density than batteries[2].
Electrodes for supercapacitors can be made of carbon, metal oxides, and polymeric materials. The most significant electrodes have been those prepared based on carbon since they have a high conductivity, are easily accessible, their costs are relatively low, and they work in a wide temperature range[2, 3]. The current commercial supercapacitor electrodes are carbon-based and use electrolytes from alkali or alkaline earth metal salts. Such carbon-based supercapacitors have been developed over time by different authors, to increase the capacitance and useful life[1].
Previous research has shown that activated carbon produced from coffee waste[4], tea waste[5], corn straw[6], cotton stem[7], hemp[8], coconut husk[9], among others, have been used as electrode materials for supercapacitors, due to the large reserves of biomass raw materials, their low cost, and good electrochemical performance. In this work, a common residue such as cassava peel is studied for its electrochemical properties and its possibility of use as electrode material in supercapacitors.
2. Materials and methods
2.1 Materials
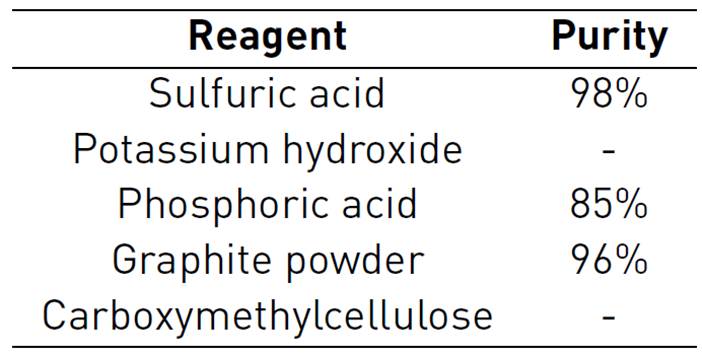
Table 1 shows the different chemical reagents used in the synthesis of electrode materials, all of them have been purchased to Merck.
Samples of cassava peel were taken from some restaurants of Barranquilla city; samples have been washed with water and dried during 12 hours with solar radiation to remove excess moisture. Dry peel samples were size-reduced in a blade mill (SIEMENS 1RF3 098-2YD90), working at 2.0 HP and 60 Hz. Powdered peel was sieved in a No. 35 ASTM mesh; the finer particles, after sieving, have been treated with a solution of sulfuric acid (0.1 M) in a mass ratio of 1:3, in order to remove the impurities by leaching, acid treatment was followed by rinsing with distilled water until a neutral pH was obtained. After 2 hours of the drying process, the pulverized peel was carbonized under nitrogen atmosphere.
Carbonization temperature was determined by means of thermogravimetric analysis. A sample was subjected to a heat treatment in a TA Instruments Modulated TGA 2950 Thermogravimetric Analysis equipment. This procedure was carried out at a rate of 10 °C/min up to 830 °C, under inert atmosphere (N2). The activated carbon was obtained through chemical activation with potassium hydroxide (KOH) solutions at 20% and 70% w/v; and phosphoric acid (H3PO4) solutions at 20% and 70% v/v. The chemical impregnation with each of the solutions was carried out in a carbon:solution ratio of 1:8 p/v for 15 hours. Additionally, impregnated samples were rinsed with distilled water to remove the excesses of the solutions until a neutral pH was obtained. Samples were dried at a temperature of 110 °C for 5 hours. The carbonization took place in a furnace for 1 hour at a temperature of 550 °C in an inert atmosphere (N2).
2.2 Characterization
Functional groups, present on the surface of the carbon, were determined by means of Fourier-transform infrared (FT-IR) spectroscopy in a spectrophotometer Shimadzu IR Affinity-1 with absorbent optics of Potassium Bromide. The spectral range of work was from 400 cm-1 to 4000 cm-1, in transmittance mode. As further analysis of the chemical composition, the elemental composition of the obtained carbons was characterized in a Sundy elemental analyzer SDCHN435 Carbon Hydrogen & Nitrogen Analyzer. Regarding to oxygen content, this must be obtained as the difference of the total amount of activated carbon and C, N, H and ash content. The ash determination was made based on ASTM D3174-04 standard in a Terrigeno D8 multipurpose electric muffle. The textural properties of the carbon are paramount characteristics regarding capacitive performance; such properties were examined by adsorbing N2 at 77 K on a Micromeritics ASAP 2020 PLUS sortometer. The surface area was determined from the adsorption curve of N2 using the BET equation.
2.3 Electrochemical performance
Working electrodes were prepared by suspending activated carbon, graphite, and carboxymethylcellulose (CMC) in a ratio of 85:5:10 (w/w) in deionized water. Such a suspension was coated on a stainless-steel mesh (300 μm), the coating was 1 cm2, the coated mesh was dried for 30 minutes at 110 °C. The working electrodes have an average mass of 0.071 g. In order to test the electrochemical performance of the electrodes, electrochemical analyses were performed by means of cyclic voltammetry and galvanostatic charge-discharge techniques in a potentiostat-galvanostat Gamry interface 1000E. A three-electrode cell, with saturated calomel reference electrode (SCE) and counter electrode (graphite), was used in an electrolytic solution of sulfuric acid (H2SO4) at 1 M. The determination of the specific capacitance may be calculated by means of Equation (1) and the energy density of the supercapacitors is determined by the Equation (2):
Where Csp is the specific capacitance, I is the current in amperes, ΔV is the working potential window, s is the sweep speed, E f and E 0 are the potential limits and m the active mass of the electrode.
Where E is the energy density and V the voltage.
3. Results and discussion
3.1 Chemical and textural properties
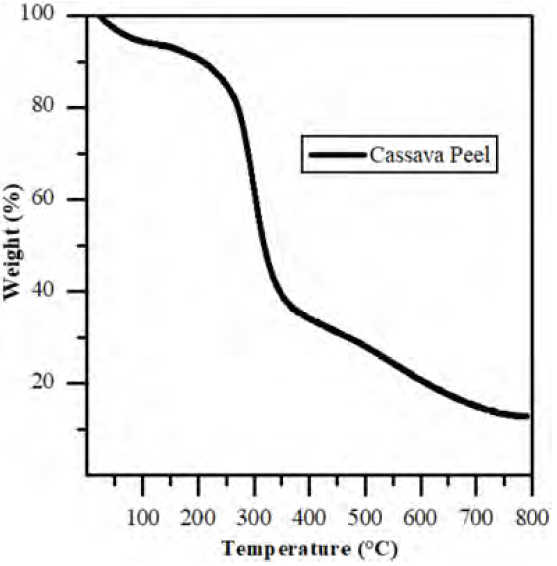
Figure 1 shows the thermogram of the precursor material (cassava peel). In the first temperature range from 20 °C to 110 °C, there is a weight loss of about 7.8% of the initial mass due to the moisture contained in the sample. A second range, which goes up to 700 °C, exhibits a loss of weight due to the decomposition of lignocellulosic materials. With this data, it was determined that the raw material’s carbonization temperature would be between a range of 500 °C and 600 °C, since in this range there is not yet a total decomposition of the material.
Four types of carbons were obtained regarding the type of chemical agent and the concentration of the impregnating agent, carbonization temperature was fixed at 550 °C, as well as carbonization time (1 h). Table 2 shows the labels used to identify the obtained carbons.
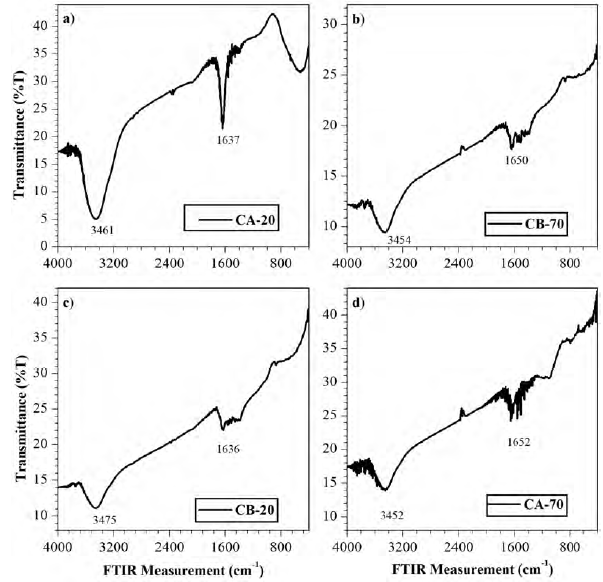
Figure 2 shows the FT-IR spectra for the activated carbon samples. It is inferred from them, that the four carbons exhibit similar characteristic bands in transmittance mode; in other words, the samples present the same functional groups. It is noticeable the presence of an intense band between 3200 cm-1 and 3650 cm-1, such a band is associated with the vibration of the stretching and flexion of the O-H bond; the band located between 1600 cm-1 and 1800 cm-1 is usually assigned to the functional group C=C, also assigned to -CH3 groups and chains of -(CH2)n- with n≥4 according to the double signal in the ranges of 2800-3000 cm-1 and their subsequent signal in the bands corresponding to the range 1385-1365 cm-1 and 730-710 cm-1[10].
Table 3 shows the results of an elemental analysis performed to the four activated carbons; there are significant differences among them in terms of carbon, nitrogen, oxygen, and ash content. However, the observed variation in the carbon amount is just apparent, since on an ash-free basis, these differences are negligible; on the other hand, nitrogen content does exhibit differences even in ash-free basis. Therefore, the treatment with both activating agents, KOH and H3PO4, does favor the decomposition of organic matter when carbonizing, but KOH treatment does not favor the retention of nitrogen functional groups after heat treatment. Ash content is high in these samples, higher in KOH treated samples than in the acid treated.
Table 3 Elemental analysis and ash content present in the activated carbons after treatment with different impregnation agents. (Percents are on a dry basis)
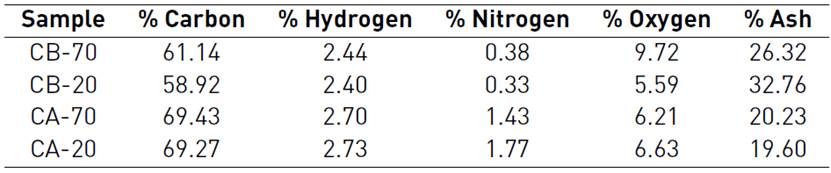
Additionally, the oxygen content was determined by the difference between the contents of carbon, nitrogen, and oxygen, as well as the ash content in the sample, it is worth clarifying that this oxygen content amounts only the oxygen from functional groups linked to the carbon, oxygen in oxides is amounted in ash content.
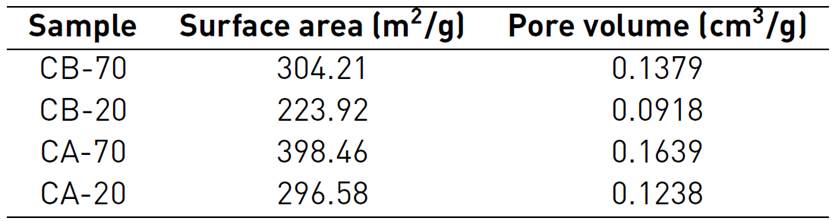
In addition to the chemical composition, and among the paramount characteristics in supercapacitors materials, are surface area and pore volume; these textural properties are summarized in Table 4. The differences observed in the surface area of the resulting carbons are related to the concentration of the chemical agent; the higher the activating agent concentration, the higher the surface area and the pore volume. This behavior has been exhibited by carbons activated with H3PO4, according to different authors[11-13], and is also present in carbons with KOH activation[8, 14]. Such behavior is due to greater interaction between the activating agent and the precursor material[11]. Some authors show that the chemical activation with phosphoric acid promotes the dilation of the precursor material, which afterward the removal of the acid, leaves the matrix in an expanded state with a porous structure; this can be evidenced with the results in this work. According to previous studies[13], this expansion is obtained in both, micro and mesopores, there is an increase in both pores’ volumes, but micropore volume gets to a constant volume while mesopores keep growing.
According to the characterization, phosphoric acid treatment allows activated carbons to be obtained with a lower ash content, a higher content of nitrogen functional groups, and a higher surface area and total pore volume than KOH treatment. In addition, a higher concentration of the acid activating agent renders a carbon with higher values of its textural properties, but a lower content of nitrogen functional groups.
3.2 Electrochemical performance
As seen in Figures 3a-d, the cyclic voltammetry curves show an almost rectangular behavior within a working window of -0.4 V to 0.6 V at four different potential rates (25 mV/s, 50 mV/s, 100 mV/s and 200 mV/s), this suggests a capacitive electrochemical behavior. Such capacitive behavior is retained with no regard of the potential rates studied, which is related to the absence of diffusional hindrance. Because of the content of functional groups, especially oxygen and nitrogen functional groups, the obtained capacitance should be the sum of pure capacitance due to the electrical double layer formation in the surface of the activated carbon, plus a kind of pseudocapacitance due to the reversible oxidation/reduction of the functional groups[15]. However, in voltammetry curves there is no evidence of any redox peak at the applied potential rates.
The surface functional groups of heteroatoms, such as oxygen and nitrogen, improve the ion adsorption process, which improves and facilitates the rapid transport of electrolyte ions within the micropores of the electrode. Furthermore, the higher electronegativity of oxygen promotes an increase in polarity of activated carbon which results in a higher hydrophilicity[4, 16-18], the higher the water affinity of the carbon, the higher the ion adsorption rates.
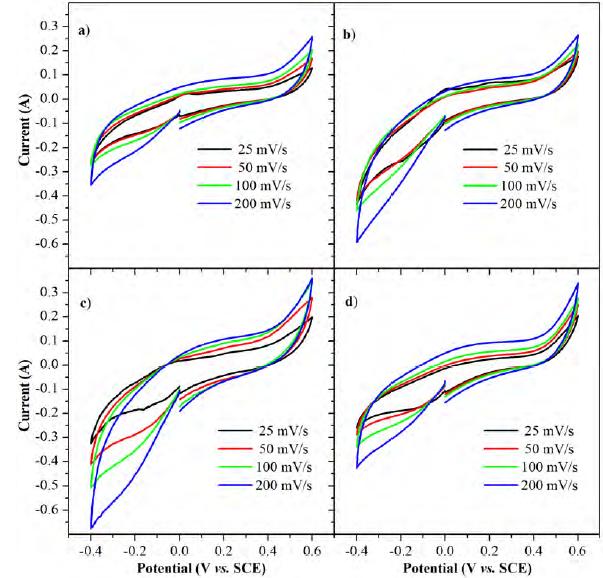
Figure 3 Cyclic voltammetry of a 3-electrode (SCE as reference and graphite as counter) configuration in a 1 M H2SO4 solution: a) CB-70, b) CB-20, c) CA-70 and d) CA-20
In Table 5, the specific capacitance and energy density data, calculated with Equations (1) and (2), are collected. At a lower potential rate, the capacitance and the energy density of the cell are higher; this is due to the higher transfer of charge during the travel of the electrolyte and its greater diffusion through the pores of the active material of the electrode. Carbons with the best performance are CA-70 and CB-70; these carbons have the highest surface area, 398.46 m2/g and 304.20 m2/g, respectively; such high surface favors the accumulation of charge at the electrode-electrolyte interface and the pore volume allows the ions to enter with a lower hindrance. Acid activated carbons exhibit higher capacitance than alkaline activated carbons at lower potential rates, this is related to a higher content in nitrogen functional groups and carbon content, which is related to a higher conductivity; it is also related to a higher surface area. However, at higher potential rates, the best behavior has been exhibited by CB-70, despite its lower contents in nitrogen and carbon, and higher ash content. CB-70 is the carbon with the highest oxygen content, such a result indicates that the oxygen functional groups have a higher effect on surface wettability, which is beneficial in order allow the fast absorption of an ion at higher potential rates.
Table 5 Electrochemical characterization by means of the cyclic voltammetry technique for the electrodes of CA-20, CA-70, CB-20, CB-70 in an electrolytic solution of H2SO4 at 1M
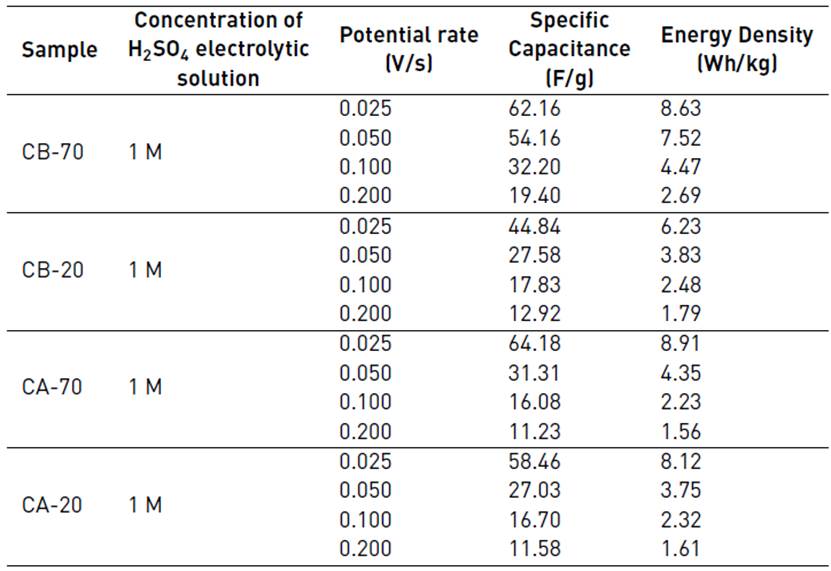
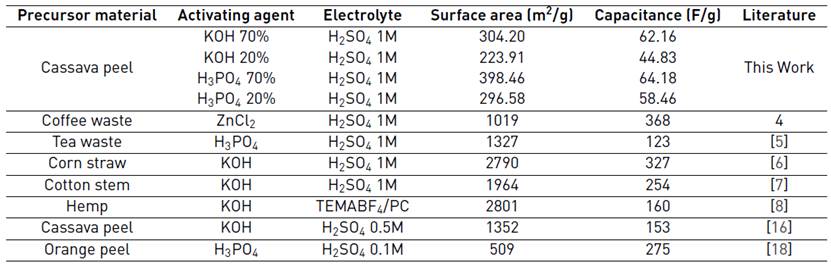
Table 6 compares the capacitive performance of carbon prepared with cassava peel with activated carbons obtained from other biomass sources. It is evident that capacitance is directly related to the surface area of the carbonaceous material. Capacitances of the cassava peel-based carbons, prepared here, are lower than those presented in the table, because they have a smaller surface area. Acid treatment leads to an active carbon with properties that allow a better performance of the electrodes in supercapacitors, and the higher the concentration of such activating agent, the higher the capacitance, since a higher surface area is achieved.
To the best of our knowledge, there is only one work[16] in which supercapacitor electrodes have been prepared from cassava peel. Our material seems to be promising, as long as the synthetic procedure gets more control and further treatment gives higher surface areas and lower ash content.
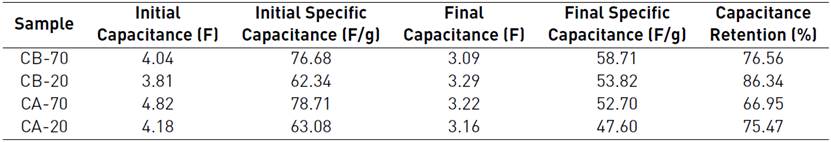
Finally, galvanostatic charge/discharge, at a current rate of 0.1 mA and through 2000 cycles, shows additional evidence on the good performance of the cassava peel-based supercapacitors. First discharge is high for the four samples, ranging from 63 to 79 F/g, as shown in Table 7, the higher initial capacitance is for CA-70 sample, 78.71 F/g, confirming what has been obtained by means of cyclic voltammetry. As expected, after some cycles the discharge capacity fades to an almost constant value, so that discharge at the 2000th cycle ranges from 47 to 59 F/g. Despite a high initial discharge capacitance, CA-70 exhibits the highest loss in capacitance, with the lower capacitance retention (66.95%) among the four samples. Such a loss in capacitance could be due to a concomitant loss of nitrogen functional groups, since these groups have been attributed as enhancers of conductivity in acid activated carbons. Post-cycling elemental analysis are necessary in order to check this hypothesis.
4. Conclusion
A combination of surface properties, surface area and pore volume, with chemical properties, presence of nitrogen heteroatoms, provides a decrease in steric hindrance, a greater polarization area, a greater wettability of the surface and an increased conductivity; this results in a better electrochemical performance of the activated carbons that were produced from cassava peel, which provides nitrogenous functional groups, at high concentrations of activating agent. The results obtained also suggest that cassava peel can become an activated carbon with promising properties for the preparation of supercapacitors; however, it is necessary to be more careful with the treatment of the raw material and the post-activation treatment to reduce the amount of ash present.