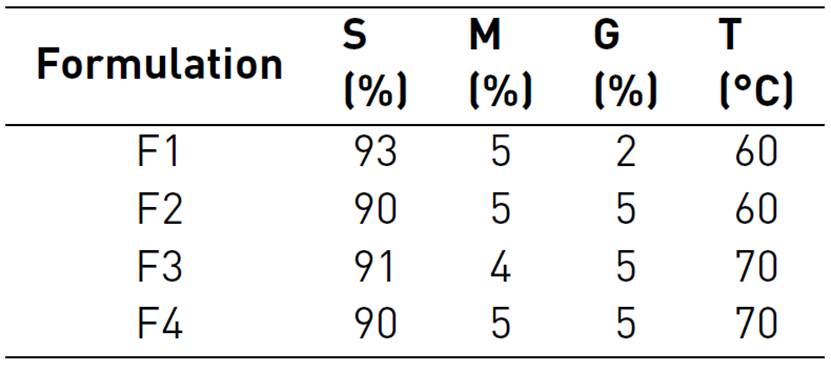
1. Introduction
Heavy metals are considered harmful inorganic pollutants for ecosystems, even when they are present in small quantities; they present high recalcitrance and persistence in the water bodies, which lead to biomagnification through natural processes, making that their concentration becomes so high reaching toxic levels for life[1, 2], and in most cases, they are difficult to remove, and if possible, their removal is costly[3].
Conventional technologies such as precipitation, oxidation, reduction, ion exchange, filtration, electrochemical treatment, reverse osmosis, adsorption, and evaporation for the removal of heavy metals have been studied, being often ineffective, especially when low concentrations of metallic pollutants are being removed, for example, chemical precipitation and electrochemical treatment are ineffective when the metal concentration is close to 100 mg / L[4, 5].
Bioadsorption is one of the most efficient wastewater treatment alternatives because of the low implementation and maintenance costs in relation to traditional heavy metal recovery treatments in aqueous effluents[3]. As for the mechanisms for the capture of metal cations, they are very varied and depend, in each case, on the metal of interest and the type of bioadsorbent material to be evaluated, and may be materials coming from microbial flora, algae, plants, biomass residuals, and agro-industrial products.[5, 6].
New friendly technologies are currently being developed, using natural polymers such as starch[7], which are capable of reducing metal ion concentrations, because these polymers have different functional groups, such as hydroxyls and amines. These groups increase the absorption efficiency of metal cations[8], and also the synergy with polymeric compounds, in the form of copolymers, formed by organic compounds (chitosan, gums, pectins, proteins, starches), and inorganic compounds such as clays[9, 10, 11].
The native potato (Solanum tuberosum subsp. Andigena), cultivated above 3,500 m of altitude, is consumed by the inhabitants of the Peruvian Andes. Potato starch is composed of amylose and amylopectin, the latter branched-chain, with a greater number of hydroxyl groups and phosphate ester[12], which gives it affinity for metal cations and chelating capacity[11, 13, 14].
In this sense, the synthesis and characterization of a biopolymer from native potato starch (Solanum tuberosum subsp. Andigena) and nopal mucilage (Opuntia ficus) are proposed, with the capacity to remove heavy metals in wastewater, allowing the development of new materials for wastewater treatment.
2. Experimental methods
2.1 Genetic material
The native potato of the Allcca sipas variety was collected in its state of commercial maturity, from the cultivation fields of the Chulcuisa Populated Center in May 2018, located at 13 ° 41 '01' 'S, 73 ° 14'20' 'W, and 3880 m of altitude, of the Andahuaylas province, Peru.
The wild nopal cladodes (Opuntia ficus indica), were collected from the area of Possoccoy in June 2018, located at 13°35'26.4" S, 73°27'00.8" W, 2500 m of altitude, from the Talavera district, Andahuaylas, Peru.
2.2 Extraction of starch and nopal mucilage
Native potato starch was obtained by hydroextraction and drying at 45 ( 2 ºC for 14 hours and sieving at 250 µm.
The nopal cladiodes were cut and immersed in water in a 1: 1 ratio; 24 hours later, the extracted liquid was treated with 96° ethanol in a 3: 1 ratio, to extract the precipitated mucilage, which was dried at 70 °C for 24 h, and grounded at 250 µm to obtain a fine mucilage powder.
2.3 Biopolymer elaboration
Aqueous solutions of 2% (p/v) (S) starch and 0.5% (p/v) (M) mucilage gelled at 70 °C were prepared and allowed to cool to room temperature; the solutions were mixed with glycerin (99.5%, Scharlau) at 40 °C according to the formulations in Table 1, in the order S - G - M, constantly stirring at 40 rpm, the mixture was placed in silicone molds. It was dried at 60 and 70 °C.
2.4 Biopolymer characterization
The functional groups of starch, crystallized mucilage, and biopolymers were identified by infrared spectroscopy with Fourier transform, in a FT-IR NICOLET 380 spectrophotometer, Thermo Scientific, USA, in a range of 4000 to 400 cm-1 with 4 cm-1 resolution, in total attenuated reflection (ATR) mode, preparing translucent crystals with 0.1% of the materials and KBr, mixed in an agate mortar.
2.5 Solubility evaluation
The method used by Dick et al.[15] was adapted, for a ratio of 1 biopolymer:100 solvent (g/mL); a solvent medium was prepared with hydrochloric acid (pH 4.7), basic with sodium hydroxide (pH 8.7), and ultrapure water. It was left to stand for 24 h, the disintegrated fraction was passed through filter paper, and it was dried at 105°C for 24 h. The solubility was expressed in terms of the percentage of dry disintegrated material.
2.6 Evaluation of metal adsorption
A solution of Al, As, Cr, Hg and Pb was prepared at 5 ppm, adjusting to pH 5 with hydrochloric acid and sodium hydroxide. 1.0 g of biopolymer from each formulation was placed in 50 mL of solution and stirred continuously at 30 rpm, taking samples at 20, 40, 60, 80, and 100 min, which were filtered and taken to an Inductively Coupled Plasma Optical Emission Spectrometer, ICP-OES 9820 Shimadzu, for metal concentration reading.
3. Results and discussion
3.1 IR analysis
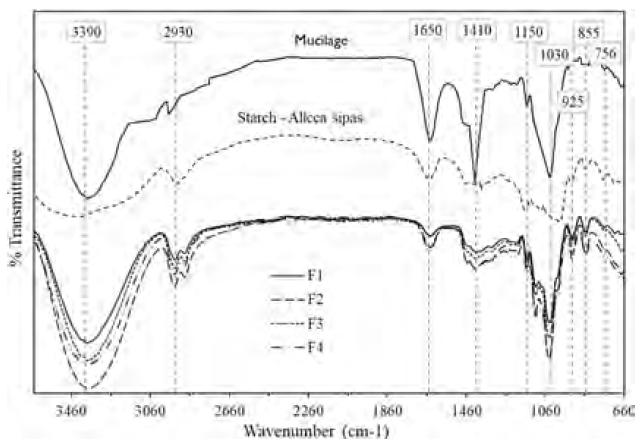
The spectra of the starch of the native potato and mucilage present intense bands around 3390 cm-1 corresponding to a -OH stretch of the alcohol and carboxylic acid functional groups; likewise, a band near 2930 cm-1 corresponding to a -C-H stretch is observed mainly for potato starch [Figure 1]. Around 1650 cm-1, formations of an intense band of a carbonyl -CO stretch of an amide were observed mainly for the mucilage; this fact is due to the presence of proteins present in the mucilage. Similarly, the presence of -CO groups of esters was observed, corresponding to bands close to 1410 cm-1. Another highly pronounced band in mucilage occurs at 1030 cm-1; it is attributed to the HC-OH stretching of cyclic alcohols, which is also present in starch, although in low signal, and would correspond to the -CO group of polysaccharides[16, 17].
On the other hand, a series of bands below 925 cm-1 was observed in the starch [Figure 1], which is characteristic for this type of material, and would be made up of the -CH and -NH stretching as well as linkage vibrational modes C-C, -CO, and C-X[18-20], with less intensity for mucilage.
The spectra of the biopolymers of formulations F1, F2, F3 and F4 [Figure 1], maintain the predominant functional groups -OH, -CO, -NH, -CH, C-OH that starch and mucilage present, nevertheless the addition of glycerin as plasticizer in the formulations would be the responsible for indicator groups near the band 1000 cm-1 [21, 22, 23]; these functional groups present in the biopolymers studied allow the capture of heavy metals due to their chelating capacity[8, 11, 13, 17].
3.2 Solubility
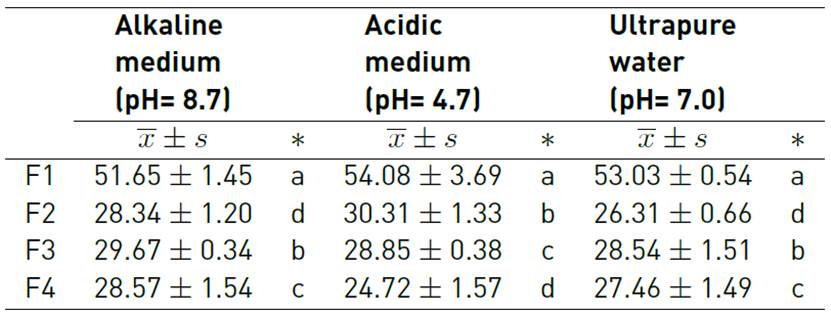
In Table 2, it is observed that the solubility in alkaline medium was 28.34 to 51.65%, in acid medium from 24.72 to 54.08%, and in ultrapure water between 26.31 to 53.03%, Dick et al.[15] reported solubility of up to 84. 5% for biopolymers made with chia seed, mucilage, and glycerol. Also, González et al.[24] reported 91.04% for nopal mucilage films, although solubility behavior will depend on the use given to the biopolymer[25].
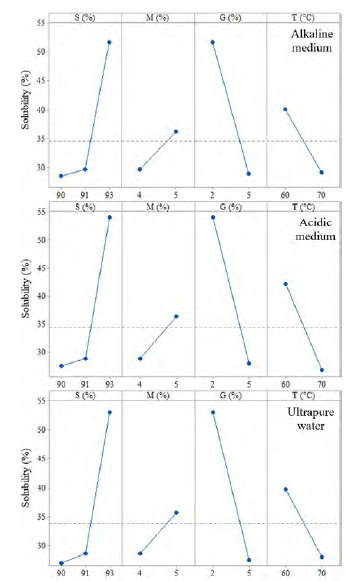
It was observed that the addition of native potato starch in the formulation of the biopolymers showed a significant effect on solubility (p-value <0.05) [Figure 2]. Thus, the F1 formulation with 93% starch showed greater solubility in all solvent media; this would be due to the amylose content, which gives it less capacity to retrograde, as well as the availability of hydrophilic groups of the starch[26, 27]; the same behavior was observed with the increase of the mucilage, due to its highly branched structure with carboxyl groups, which gives it greater ability to link and interact with the carboxyl and oxydryl groups of starch[12, 28]; however, the addition of glycerin considerably reduces the solubility [Figure 2]. This is because the glycerol acts as a plasticizer, giving it greater resistance to being dissolved[28, 29]; this plasticizing capacity is higher when the temperature increases.
3.3 Metal removal
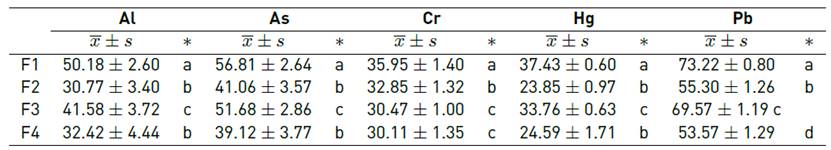
It was observed that the biopolymers presented good affinity for heavy metals, for a contact time of 100 min, removing between 23.85 to 37.43% of Hg, 30.11 to 35.95% of Cr, being the Pb the one that presented greater removal between 53.57 to 73.22% [Table 3]. On the other hand, it was observed that the formulation F1 reported better capacity of metal adsorption, which would be related to the high solubility that presents.
The addition of potato starch and mucilage had a significant effect (p-value < 0.05) in increasing the adsorption capacity of heavy metals, while the increase in glycerin and the drying temperature considerably reduce this capacity of the biopolymer.
The fact that biopolymers have a good affinity for metals is due to the availability of functional groups with a chelating capacity[30], forming complexes with the metal cations, or by the capacity to generate ion exchange[8, 11, 13, 14, 31].
Biopolymers have been observed to have unprotonated hydroxyl groups, which interact in the S-M-G coordination systems with metal ions. On the other hand, the manifestation of the amino and acetyl groups, coming mainly from the mucilage, allows them to link easily with the transition orbitals of the metals[32], so that they are fixed to the surface of the biopolymer in the form of metal complexes. On the other hand, the fact that biopolymers have a better affinity for Pb could be attributed to the atomic radius and the oxidation number[33].
However, adsorption is conditioned by the pH of the solution, since in highly acidic aqueous media, the chelating groups are protonated[34, 35], destroying the metal-binding bond[36-38]. In this sense, the modification of the pH would improve the adsorption capacity of the biopolymers under study, although at alkaline pH, the metals usually precipitate.
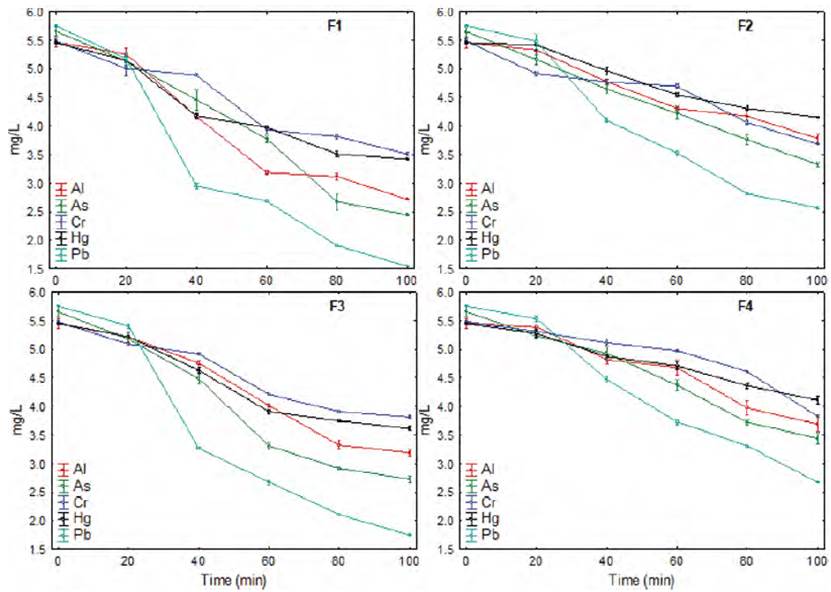
Similarly, during the adsorption of metals over time, a constant decrease in concentration is observed, with a similar behavior for all the biopolymer formulations [Figure 3], although at longer contact times, the increase in removal is minimal[9, 13, 39, 40,41].
4. Conclusions
The use of organic materials such as native potato starch and nopal mucilage, used for the synthesis of biopolymers, makes it possible to improve the chelating conditions of the functional groups they present, predominantly -OH, -C-O-, -NH-, -C-H-, -C-OH. This allows the removal of up to 50.18% Al, 56.81% As, 35.95% Cr, 37.43% Hg and 73.22% Pb, for a contact time of 100 minutes at pH 5.0, being positively influenced (p-value < 0.05) by the addition of starch and mucilage. In that sense, the synthesis of biopolymers based on native potato starch and nopal mucilage shows a promising horizon for the removal of heavy metals, especially in mining wastewater.