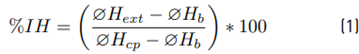1. Introduction
The spread of diseases caused by pathogenic microorganisms is a public health risk factor of great concern [1]. Bacteria commonly implicated in foodborne illness (FD) include Staphylococcus aureus, Escherichia coli, and Listeria monocytogenes species or the Salmonella, Campylobacter and Shigella genera [2,3], and the resistance they have developed to antimicrobials has been increasingly recognized as a major problem constituting a health hazard that transcends international boundaries [4].
Multi-drug resistant (MDR) Shigella strains, particularly those resistant to fluoroquinolones and broad-spectrum cephalosporins, have exacerbated this global health burden, leading to increased morbidity, mortality, and treatment costs [5]. Shigella has four serogroups, S. dysenteriae, S. flexneri, S. boydii and S. sonnei, which cause more than 164,000 deaths per year, with 55,000 of these among children under 5 years of age. It is identified as one of the most important agents of diarrhea according to the World Health Organization (WHO), mainly in developing countries [6], representing 11% of all deaths from diarrhea globally[7]. Shigella also causes shigellosis or bacillary dysentery, which is accompanied by a high fever, neurological disorders, and muco-hemorrhagic dysentery [8].
Lactic acid bacteria (LAB) found in dairy products such as milk drinks, yogurt, cheeses (fresh and mature), meats and their products, and in certain vegetables, have been present in the human diet since ancient times [9]. According to research conducted by [10], LAB belong to the phylum firmicutes, which distinguishes about 20 genres, the main ones of which are Lactococcus, Lactobacillus, Streptococcus, Leuconostoc, Pediococcus, Aerococcus, Carnobacterium, Enterococcus, Oenococcus, Tetragenococcus, Vagococcus, and Weisella; LAB comprise a diverse group of Gram-positive, non-spore-forming, non-motility and coco bacillus-shaped organisms and catalase deficiencies [10]. In terms of fermentation, LAB are classified as homofermentative ( they produce lactic acid) and heterofermentative (they produce lactic acid and other substances), and, in terms of growth temperature, they are classified as mesophilic and thermophilic [10], for which the ideal incubation temperature is 20-25 °C and 40-45 °C, respectively [10].
LAB are characterized by the production of antimicrobial substances (lactic and acetic acids, metabolites), [11] and bacteriocins, which are a family of antimicrobial peptides produced by bacteria through ribosomal synthesis that can act as helper peptides for probiotic strains in the gastrointestinal tract [12]. Given these properties, the UN Food and Agriculture Organization (FAO) and the World Health Organization (WHO) have defined probiotics as "living microorganisms which, when administered in appropriate doses, confer beneficial effects on the health of the consumer" [13]. In this vein, it is important to perform in vitro antibiogram tests, to discover the susceptibility capacity of LAB against antibiotics, in order not to create multi-resistance if they are applied together.
LAB produce several antimicrobial components which inhibit the growth of relevant sporadic organisms. Currently, the production of bacteriocins by bacteria of the Lactobacillus genus has been successful in foods such as meat to control certain pathogenic microorganisms, such as Salmonella spp. and E. coli [14]. It has also been demonstrated that, in food production technology, they perform the following functions: formation of acid taste, inhibition of pathogenic organisms, jellification of milk, reduction of lactose content, production of gas required for the formation of eyes in cheeses, and they are widely used as probiotics [21].
Treatment with antibiotics is not always effective, and in many cases, causes resistance. It is thus important to find natural techniques to control and reduce infections and / or diseases caused by this pathogen. With these requirements established, the study was based on evaluating the antimicrobial effect of LAB strains from regional products from Colombia against the pathogen Shigella sonnei ATCC 25931 and their susceptibility to antibiotics.
2. Experimentation
2.1. Strains
Bacterial strain Shigella sonnei ATTC 25931
The pathogen of the reference strain Shigella sonnei ATCC 25931 (KWIK STIK, France, Europe) was activated and inoculated into the standard method-P.C.A. agar (Plate Count Agar, Conda, Spain) using the triple striae method, in order to obtain a pure culture. The plate was incubated at 37 °C for 24 h.
Pathogen growth count
P.C.A. agar (Plate Count Agar, Conda, Spain) was seeded and then incubated at 37 °C for 24 hours; a portion of the growth was deposited in 9 ml of peptone water (Merck KGaA, Germany), homogenizing it until reaching the 0.5 McFarland standard. This was followed by serial dilutions until 10-9, and 100 µl were seeded on the surface. The plates were incubated at 37 °C for 24 hours. In all cases, this was carried out in duplicate, and phenotypical identification was conducted using the Gram stain method.
LAB Strains
LAB strains (L, D, JC, 12) preserved at -20 °C from the collection at the Food Microbiology Laboratory (Universidad Surcolombiana, Neiva-Huila) were activated. LAB L and D come from the research project[15], strains from semi-dry coffee fermentation (without water) with 0 and 24 hours of fermentation, respectively. LAB 12 was derived from the research project [16], and originates from the product (quesillo) under refrigerated storage conditions of 4°C. LAB JC came from mother's milk, which was obtained under aseptic conditions and stored at 4°C. Surface culture was conducted in Man, Rogosa, Sharpe (MRS, Conda, Spain) agar medium incubated at 37 °C for 24 hours. Pure isolates were subsequently obtained using the triple striae culture technique. Finally, surface culture was performed on MRS agar, incubated at 37°C for 24 hours. Phenotypical identification was conducted using the Gram stain method.
2.2. Evaluation of the antimicrobial activity of lactic acid bacteria (LAB)
The effect of LAB was evaluated in the presence and absence of cells against the pathogen Shigella sonnei ATTC 25931. In all cases, this was performed in duplicate.
Disk diffusion method
The disk diffusion technique was used to evaluate the inhibition of pathogens in the presence of LAB cells with an acidic pH. A 7 mm diameter disc was cut from each strain (JC, L, D, 12) using a sterile biopsy punch [17]from 24 hours mass culture on MRS agar. The discs were deposited in PC agar medium where the Shigella sonnei ATCC 25931 culture was inoculated on the surface and adjusted to the 0.5 McFarland standard. A well was made for the positive control, to which 50 µl of the antibiotic Ceftriaxone (Nirlife-Aculife, India) was added. The plates were incubated at 37 °C for 15 hours [Figure 1]. The inhibition zone was measured according to [17].
Well diffusion method
The well diffusion technique was used to evaluate the inhibition of pathogens in the absence of LAB cells with acidic [4-5] and neutral (7) pH. In the neutral pH test, this was verified using a tape measure and adjusted with 0.1 N sodium hydroxide-NaOH (Merck, Germany).
Initially, a portion of the LAB cells (from a 24-hour mass culture on MRS agar) was deposited in tubes containing Man, Rogosa, Sharpe broth (Oxoid, England), taking them to the 2 McFarland standard, incubated at 37 °C. After 24 hours, the liquid with extracellular content from LAB culture was added to 1.5 ml tubes (Eppendorf, China). These were then centrifuged at 6000 rpm for 10 minutes. The supernatant was separated to obtain the cell-free substance to acid pH. For neutral pH evaluation, NaOH was added to the tubes with the supernatant until it reached pH 7. Five 7-mm diameter wells were sectioned using a sterile biopsy punch [17]on PC agar where the Shigella sonnei ATCC 25931 culture was spread on the surface at 0.5 McFarland standard; 50 µl of each supernatant was added to the wells, and the antibiotic Ceftriaxone was used as a positive control. The plates were arranged in incubation at 37 °C for 15 h [Figure 2]. The inhibition band for the LAB strains was measured according to [17].
2.3. Antibiograms
LAB (JC, L, D, 12) were evaluated using the well diffusion technique with the antibiotics Penicillin G. Benzatine 1,200,000 U.I. (Vitalis, Spain), Ceftriaxone 1,000 mg (Nirlife-Aculife, India) and Ciprofloxacin 500 mg (Biochemist Pharma S. A, Colombia) in MRS agar medium, where three wells of 7 mm diameter were cut using a sterile punch [17]. Fifty µl of the respective antibiotic was added to each well. Finally, they were incubated at 37 °C for 15 hours. The inhibition zone caused by the antibiotics was measured according to [17].
2.4. Calculation of the inhibition zone (%)
Equation (1) below was used to determine the inhibitory capacity of LAB and their susceptibility to antibiotics [1].
Where, ØHext: extract halo, ØHb: White halo (7 mm), ØHcp: positive control halo.
2.5. Statistical analysis
An analysis of multifactorial ANOVA variance with a 95% confidence level was performed to determine the results of LAB antimicrobial activity in the presence and absence of cells obtained in the laboratory. This determined how the factors involved (LAB and diffusion methods; discs and wells) significantly affect the percentage of inhibition, allowing a comparison to be made between them. The statistical analysis was performed using the StatGraphics software (Centurion XVI Version 16.1.03).
3. Results and discussion
3.1. Strains
Shigella sonnei ATTC 25931 bacterial strains
Isolated colonies were obtained from the suspension (0.5 McFarland standard). These were circular with irregular edges; they were concave, opaque, translucent, whitish, milky, smooth and with 1 to 3 mm thick diameters. Bacilli were observed, identifying Gram-negative bacteria.
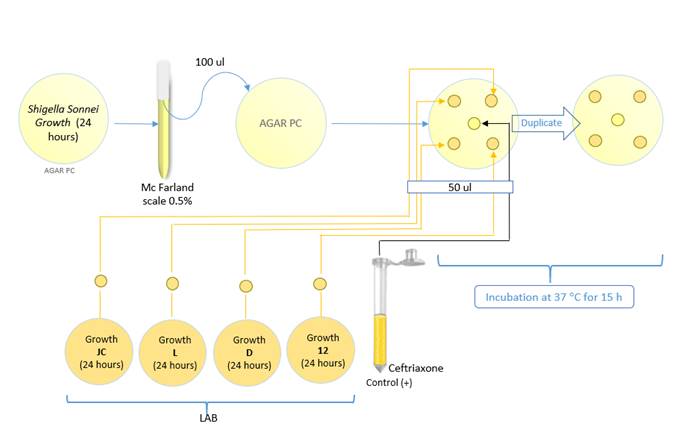
Figure 1 Diagram of the antimicrobial activity test with LAB cells against Shigella sonnei ATTC 25931
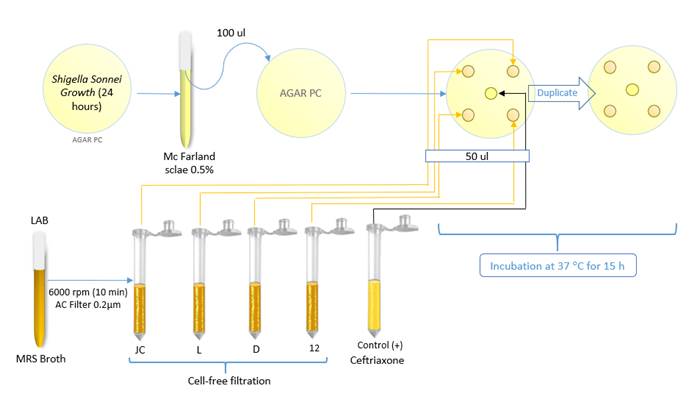
Figure 2 Diagram of the antimicrobial activity test in the absence of LAB cells (acid and neutral pH) against Shigella sonnei ATTC 25931
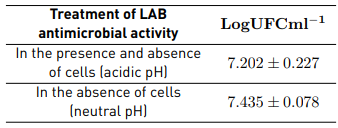
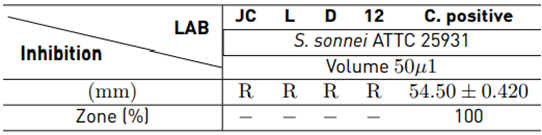
Table 1 shows the concentrations of the bacterial strain Shigella sonnei ATTC 25931 for antimicrobial activity.
LAB strains
The native strains JC, L, D, and 12 have the following macroscopic and microscopic characteristics:
Strain 12 has rounded white colonies on its periphery, it is milky-colored in the center, with a diameter of 3 mm. Bacilli were observed, identifying the bacteria as Gram-positive [16].
Strain L has opaque white colonies, round in shape with a diameter of 8 mm, and concentric rings. Bacilli were observed, identifying the bacteria as Gram-positive [15].
Strain D has milky white colonies, with a diameter of 4 mm and a defined circular shape. Short bacilli with smooth, ungrouped ends were observed, identifying the bacteria as Gram-positive [15].
The JC strain has colonies with circular, concave, yellow, milky characteristics, with 1 mm diameters. Cocci were observed, identifying the bacteria as Gram-positive.
3.2. Evaluation of the antimicrobial activity of lactic acid bacteria (LAB)
Disc diffusion method
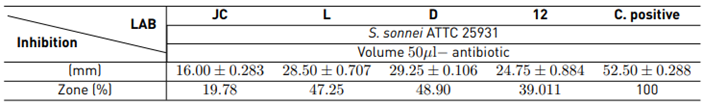
Table 2 presents the results obtained in the evaluation of LAB in the presence of cells:
The formation of inhibitory halos in LAB is evident in all native strains: the D and L strains are the ones with the largest inhibition zone with 48.90% and 47.25%, respectively. The action of the positive control is confirmed with a halo of approximately 52.50 mm in diameter.
Well diffusion method
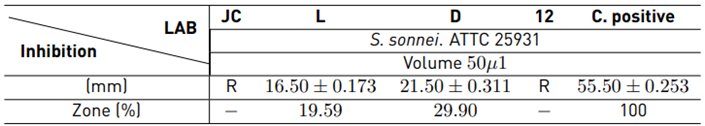
Table 3 presents the results obtained from the evaluation of LAB in the absence of cells (acidic pH).
Organic acids in strains D and L inhibited Shigella sonnei ATTC 25931 with an inhibition zone of 29.90% and 19.59%, respectively. Metabolites from strains JC and 12 did not inhibit the pathogen Shigella sonnei. It was noted that each LAB produces different metabolites, and antimicrobial activity is considered to be strain-dependent and not a characteristic that is generalized across the LAB group. The positive control action is confirmed with 55.50 mm diameter halos.
The effect of bacteriocins against Shigella sonnei ATCC 25931 was studied when cell-free supernatants were obtained under pH 7, and the results showed that the LAB evaluated did not contain bacteriocins that inhibited the growth of the pathogen under study. Normally, bacteriocins are produced by bacteria that present antibacterial activity, which becomes more effective in gram-positive bacteria [18].
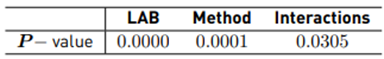
Table 5 shows the P-values obtained by multifactorial ANOVA for the LAB and method factors; these values prove the statistical significance of each of the factors. All 3 P-values are less than 0.05, i.e., these factors have a statistically significant effect on %IH at a 95.0% confidence level. It follows that the inhibitory effect of LAB versus Shigella Sonnei ATCC 25931 shows significant differences (p<0.05) between LAB and the methods.
By observing the behavior of each of the LAB with respect to the positive control with the diffusion methods, it was found that the disk-diffusion method presented the LAB with a greater degree of inhibition; the native strain D (coffee strain) presented its strongest inhibition behavior in the disc method (presence) and the well method (absence). Similar results were found by [18] with strains isolated from traditional Chinese fermented food, their free-cell supernatant from L. paracasei M5-L, L. rhamnosus J10-L, L. casei Q8-L inhibited S. sonnei ATCC 25931. In theory, LAB and yeasts are naturally found in coffee and they are microorganisms of dietary interest as these strains show in vitro antimicrobial activity against the pathogen under study. They could be considered to be probiotic LAB, although more FAO-recommended tests are required to confirm this [19]. It should be noted that the antimicrobial effect of native strains of LAB against the pathogen Shigella sonnei ATCC 25931 depends on these strains. It cannot be said that all native strains in cheese, coffee and breast milk have this antimicrobial activity against the reference strain Shigella sonnei ATCC 25931; the tests carried out are qualitative; however, they may be useful in future in vivo research.
The results obtained for LAB against Shigella sonnei ATCC 25931 in pH 7 conditions are related to the findings obtained by Amorocho-Cruz, where LAB supernatants studied at pH 7 had no antagonistic effect against the Salmonella enteritidis and S. typhimurium strains studied. These results coincide with other research concluding the importance of the presence of cells to adhere to and colonize the epithelial cells of the stomach antrum [19]. Another study also showed that Pediococcus pentosaceus strains isolated from idli batter produce lactic acid and another antimicrobial active substance at pH 6, which present effective inhibition against Gram-positive and Gram-negative intestinal pathogens (Escherichia coli, Listeria monocytogenes).
However, when exposed to a different treatment (Protease) the antimicrobial activity was lost [20], thus demonstrating that the antimicrobial substance is bacteriocin in nature, as shown by [21].
Studies on H. pylori showed that most of the LAB evaluated presented inhibitory activity in the presence and absence of cells. Other authors such as Smaoui et al. have further evaluated the acting compound. They establish that the TN635 strain has an antimicrobial compound of protein origin, which is stable when heated and active between pH 3 and 11, with a molecular mass of approximately 4 KDa, and with a bactericidal effect against Listeria ivanovii BUG 496 [22]. These studies have identified that the compounds of these LAB are more active at acidic pH than in neutral conditions, where it is assumed that lactic acid has been generated, but a molecular identification is needed to identify whether it is only this acid that is present or whether other organic acids are present also.
In a study on the characterization and probiotic potential of lactic acid bacteria isolated from Guirra sheep's milk, it was found that this product contains lactic acid bacteria that show antimicrobial activity against the pathogens H. pylori, S. typhimurium and S. enteritidis [19]. Other authors similarly report having found lactic acid strains obtained from foods that inhibited the growth of these pathogens [23,24]. This was the case in the study of antimicrobial activity of 131 lactic acid bacteria strains isolated from Guirra dairy sheep in Salmonella strains using agar diffusion tests (with and without LAB cells). It was shown that a high concentration of supernatant is required to observe an inhibitory effect using the agar diffusion test, suggesting that, in vivo, this antimicrobial effect would affect pathogenic bacteria on the epithelial surface only if high amounts of antimicrobial substances are produced. Therefore, the antibacterial activity of these strains of lactic acid bacteria seems to result from the activity of different factors with different potencies and spectrum of action [25].
An in vitro LAB-related study conducted in 2018 by Bin Masalam et al. involved 46 LAB from raw and fermented milk in Saudi Arabia against seven bacterial indicator strains; the results indicated antibacterial activity of the cell-free supernatant (CFS) of 25 (54.35%), 38 (82.61%), 33 (71.72%), 31 (67.39%), 11 (23.91%), and 38 (82.61%) LAB strains against E. faecalis, E. coli, Salmonella spp., Shigella sonnei, S. aureus and Listeria monocytogenes, respectively [26].
According to [27], pretreatment using Lactobacillus rhamnosus and L. acidophilus, reveals that the cells of these bacteria interfere with the adherence of shigella to epithelium cells, attenuating inflammatory responses during aggregate pretreatment. Lactobacilli also stimulate the immune system. It is very useful to know that the LAB isolated from products from Southern Colombia presented an inhibitory effect against the reference pathogen, evincing the viability of these strains under different conditions. For example, the coffee strains at different times of fermentation (D and L), the quesillo strain isolated characterized as psychrophilic (12) and the strain isolated from mother's milk, whereby according to the mother, her child did not develop any illnesses during the whole period of breastfeeding. This supports Hippocrates' statement “Let your food be your medicine and your medicine be your food.”
3.3. Antibiograms
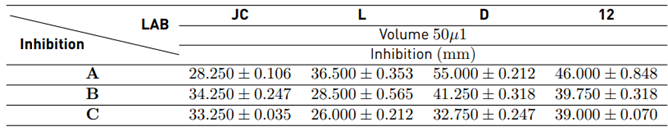
Table 6 presents the results of the microbiological test to determine the susceptibility of LAB to 3 types of antibiotics.
The most sensitive LAB was D with the antibiotic Penicillin G. Benzathine and Ceftriaxone, respectively, and LAB L showed less inhibition with the antibiotics Ceftriaxone and Ciprofloxacin, respectively. Ciprofloxacin is a quinolone antibacterial agent that interferes with DNA replication; penicillin is a β-lactam antibiotic that inhibits cell-wall biosynthesis. Shigella spp. mainly influences susceptibility to the slow penetration of β- lactams and low permeability of hydrophilic antibiotics such as penicillin. Ceftriazone is used to treat ciprofloxacin-resistant Shigella strains [28]. LAB are carriers of chromosomal encoded multidrug-resistant efflux pumps that can be transferred to pathogens or give resistance to compounds produced during fermentation processes causing food spoiling [28]. In general, LAB were susceptible to in vitro inhibition by the concentration of antimicrobials (A, B, C), showing that antibiotics have inhibitory effects on them; LAB are viable, selected microbial dietary supplements that, when introduced in sufficient quantities, beneficially affect the human body by their effects on the intestinal tract [29,30]. The suggested concentration of LAB is in the 106-107 CFU/g range [31,32]. Thus, LAB may be used to combat infection with the pathogen Shigella sonnei ATCC 25931, as they exhibit non-transfer of resistance to surrounding pathogens.
It should be noted that antibiotics are bactericidal/bacteriostatic molecules that control bacterial infections; however, their misuse favors multi-resistance or therapeutic failure in the case of naturally resistant bacterial strains, thus generating a health risk [33].
4. Conclusions
The LAB strains isolated from the coffee, cheese, and breast milk fermentation processes exhibit probiotic properties as they were able to inhibit Shigella sonnei ATCC 25931 growth under in vitro conditions, due to the presence of the cells, their components, and lactic acid. This inhibitory effect depends on the lactic and the pathogenic strains, and cannot be generalized to a species. It is important to be able to incorporate these LAB (coffee fermentation process) in food; however, before developing this process, it is necessary to identify the food matrix and evaluate the viability of each LAB. It is also important to evaluate the behavior of the LAB strains in gastrointestinal conditions and their adhesion to the epithelium to obtain beneficial results in the human body.