1. Introduction
Biomass was humanity's most important energy source until the beginning of the industrial revolution, when it was relegated to second place due to the massive use of fossil fuels[1]. However, environmental problems and climate change caused by the high consumption of fossil fuels have renewed interest in ethanol production and its use as an alternative fuel in transportation [2].
Cocoa mucilage is a source of sugars released as exudate, composed of sugars such as gums and pectin [3]. This residue can cause problems due to its accumulation, which attracts insects and rodents through fermentation [4]. Cocoa mucilage is fermented anaerobically, where yeasts and lactic acid bacteria predominate. These metabolize sugars and citric acid in cocoa mucilage, generating acetic acid and ethanol [5].
In the aerobic fermentation of the mucilage, the production of metabolites such as ethanol and organic acids is originated by the action of microorganisms in the natural environment, such as yeasts, lactic bacteria and acetic bacteria. [6]. The fruits (pods) of cocoa are berries with a thick and hard exocarp (shell) between 7 and 20 mm wide, which serves as protection for the beans arranged along an embryonic axis and surrounded by a mucilaginous liquid [7]. In addition, cocoa beans are surrounded by mucilage containing 10-15% sugar, 1% pectin and 1.5% citric acid, which is necessary to produce alcohol and acetic acid in the fermentation of almonds [8].
To understand the research in which yeasts are responsible for transforming sugar into alcohol, we start by their definition that they are unicellular ascomycetes or basidiomycetes whose vegetative growth results predominantly from budding or fission and that they do not form their sexual states [9]. Among the best-known yeasts for this process, the Saccharomyces genus has received most of the attention of the scientific world due to its usefulness as a fermenter; therefore, species such as S. Cerevisiae (SC), S. Bayanus, S. Pastorianus and S. Paradoxus, among others, are well known in the fermentation field [9]. Furthermore, yeasts are the most widely used microorganisms in ethanol production due to their productivity, low production of inhibitors, and ease of separation after fermentation [10]. For this reason, the application of Saccharomyces genus yeasts is essential in cocoa fermentation. It acts in the fermenters for 7-8 days and allows the grains to be separated from the mucilaginous pulp that protects them [11].
Also, it should be noted that ethanol is a net energy source, easily storable, with a high oxygen content (35%) and clean combustion; it is considered to have an excellent potential for application as fuel [12]. Furthermore, a renewable resource is biomass generated by photosynthetic organisms (autotrophs), which store energy in the form of sugars that can be transformed into ethanol through fermentation [13]. Therefore, the research focused on obtaining bioethanol from cocoa mucilage through an anaerobic fermentation process with the application of different concentrations of yeast (Saccharomyces cerevisiae).
Biofuel elimination from waste such as cocoa mucilage would help partially reduce the consumption of coal, gas and oil used by countries. For example, in 2002, 8,034 million tons of oil were consumed, representing a 1.3% increase in consumption compared to 0.3% the previous year. Industrial development is the factor that most influenced the rise in fuel consumption [14]. Also, the development of new energy sources has been growing in each of the stages that have marked the development of the different sectors of the world economy, which allows the introduction of new energy sources [15].
2. Materials and methods
2.1 Materials
The following work was carried out in the Quevedo State Technical University laboratories, located on the Vía a Santo Domingo, km 1.5 of Quevedo canton. The raw material to produce bioethanol from cocoa mucilage combined with different concentrations of yeast was purchased in Quevedo (see Figure 1].
Bioethanol was obtained through the fermentation process of the mucilage of two varieties of cocoa: Nacional and CCN51, which were collected in seed and liquid form in a cocoa marketer located in Valencia, Los Ríos. The commercialised cocoa is deposited in boxes made of wood, with holes that allow the separation of the solid and liquid parts of the cocoa beans. The liquid form is called mucilage, and the solid state is the cocoa beans. The raw material was subjected to a pH and total solids initial analysis and later used in fermentation.
2.2 Experiment methodology
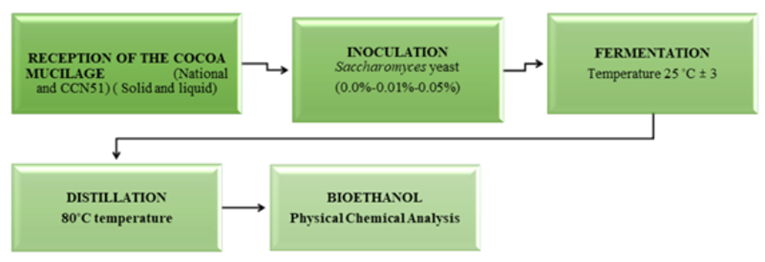
Reception: The liquid cocoa and the solid form found in the wooden fermenters were collected. The solid and liquid state of the cocoa was allowed to separate. These cocoa components were measured by pH and total solids.
Inoculation: Yeast concentrations (0%, 0.01%, 0.05%) Saccharomyces cerevisiae were applied to each experiment and homogenised to ensure adequate yeast distribution at 25°C in 4000mL of cocoa mucilage. In this phase, the sugars in the cocoa mucilage are converted into ethanol. A mechanism is placed to control the dioxide carbon output generated by the mucilage fermentation of each experiment, containing a pH of 4.1±0.5.
Fermentation: The fermentation process is anaerobic; it occurs without oxygen (some nutrients were used for the fermentation process). An airlock CO2 valve was placed to control the carbon dioxide output generated by the fermentation of the mucilage in each experiment. Each experiment contained a pH of 4.1±0.5; in this phase, the sugars in the cocoa mucilage are converted into ethanol.
Distillation: It was carried out in a simple distiller, with an 18 litres capacity at 80°C, which is automatically regulated, and a condensation system connected to a cooling tower for refrigerant recirculation for approximately 2 hours for each experiment. Subsequently, the corresponding chemical-physical analyses were carried out: turbidity, density, specific heat, bioethanol production, pH, total solids, acidity, and alcoholic degrees. The simplified process flow is shown in Figure 2.
pH measurement was performed at 25 °C (room temperature) using an isoLAB Table digital pH meter/potentiometer, calibrated before use with pH 4.0 and pH 7.0 buffer solutions, applying the AOAC 981.12.16 method [16].
Titratable acidity was determined by titration by taking 10 ml of the sample with 0.1 N NaOH and using phenolphthalein as an indicator. Then, it was determined according to the official method of the Ecuadorian Technical Standard INEN 381, applying the formula based on the predominant acid in the sample and the IFU No. 3 (1968) ISO 750:1998 method [17].
Total solids content was determined by AOAC 983.17 EN 12143 (1996) IFU Method No. 8 (1991) ISO 2173: 2003, with an ATC refractometer 0-32% Honey Sugar Brix Test B8W7 [18].
For alcoholic degrees (°GL), 90 mL were measured for each experiment using an alcohol meter. °GL was taken in an "AQUEOUS LABORATORY", which is the degree of a pure hydroalcoholic mixture indicated by the Gay Lussac centesimal alcohol meter. According to the INEN 340 "Alcoholic Beverages" standard, the ethyl alcohol content is determined by the glass breathalyser method [19].
Density. To determine the density of a liquid (ethanol), the pycnometer method was applied, according to the Ecuadorian Technical Standard for Alcoholic Beverages, determination of relative density INEN 349 [20].
Turbidity. This analysis is carried out in a "HACH TURBIDIMETER" equipment, which returns the values in NTU (Nephelometric Turbidity Unit) for which the Mandatory Ecuadorian Technical Standard 2262 is applied [21].
Specific heat. It is determined through a "TBCF" Bomb Calorimeter, applying the test method T213-2008, model BLS-384. The temperature increase is according to the heat of each sample which is constantly moved with a stirrer, and then the solution reacts at 6 volts and 1 amp [22].
Combustion. La potencia de combustión se determinó con las muestras con un contenido de 80°GL. This analysis was carried out by combining ethanol with gasoline and testing in a Datsun 1200 combustion engine (1.0-litre (988 cc) A12 engine) for 60 minutes in a proportion of 10% bioethanol and 90% bioethanol. Commercial gasoline, and 5% bioethanol and 95% commercial gasoline [23].
2.3 Statistical experiment
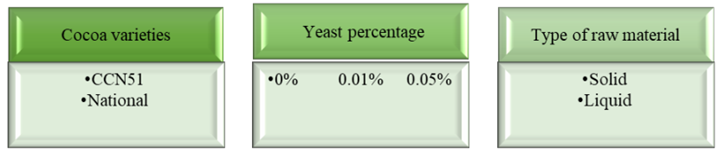
Through the results obtained, a completely randomised design (ANOVA) was applied with a factorial arrangement of 2*3*2 for the varieties of cocoa (National, CCN51), percentage of yeast (0%, 0.01% and 0.05%) and type of raw material (solid or liquid), as shown in Figure 3.
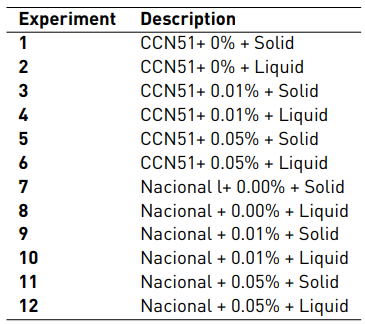
The description of each experiment, which consisted of 12 experiments and two repetitions for a total of 24 experimental units developed sequentially, is found in Table 1. The data were analysed in the Stat Graphics Centurion XVI program.
3. Results
The results of each experiment are presented in Table 2, which consists of two repetitions, the same ones in which the following analyses were carried out: pH, total solids, acidity, alcoholic degrees, turbidity, specific heat, density, and bioethanol production. Afterwards, the data is entered into the statistical program to identify the differences between each experiment.
Regarding the physicochemical data of each experiment, the same ones that were subjected to a Tukey test at a significance of 5% are detailed in Table 3. The experiment with the best characteristics stands out: CCN51 + Yeast 0.05% + solid, which allows for differentiation in the analyses such as pH, total solids, acidity, °GL, turbidity, specific heat, density, and bioethanol production.
Bioethanol results, with the application of cocoa mucilage in combination with different yeast concentrations, are in Table 3. Physicochemical analyses of bioethanol presented representative values in some parameters. Considering that the properties evaluated are not all the parameters that define the bioethanol quality is essential. These parameters are grouped into physicochemical criteria, which depend on many factors and their interactions. They are linked to the main physical components: chemical products of the mucilage (total solid, acidity), so that the fermentation develops.
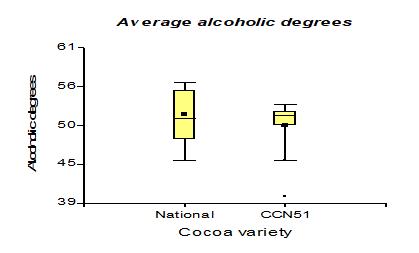
Table 3 shows the data regarding pH with 4.35 for the Nacional variety, while in CCN51 a value of 4.15 was obtained. Regarding total solids, the experiment with the highest value is found in the CCN51 with 15.05% and the Nacional with 10.06%. Regarding titratable acidity, the highest value was observed in CCN51 at 12.4%, and in Nacional the value was 9.59%. In alcoholic degrees, it was found that the mucilage of the Nacional variety presented a high percentage of 51.58% and CCN51 50.0%, which are positive values.
In the titratable acidity data, there was a significant difference in the interaction of the cocoa varieties with the percentage of yeasts, and its coefficient of variation was 21.62. In alcoholic degrees, a significant difference was established in yeast percentage when applying 0.05%.
Table 3 Physicochemical analysis of bioethanol from cocoa mucilage
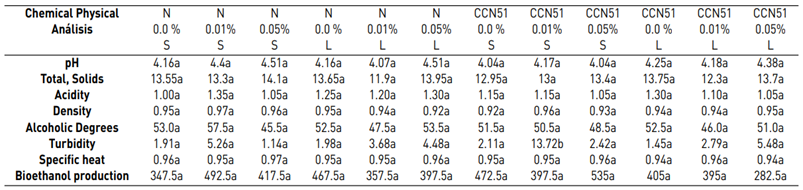
D.E. (CCN51 0.05) pH 0.04, total solids 2.69, Acidity 0.21, Density 0.01, ° GL 4.95, Turbidity 0.72, Specific heat 0 and Performance 21.21. (P <0,05) N 0.0% S = Nacional, 0.0 yeast, Solid mucilage N 0.01% S = Nacional, 0.01 yeast, Solid mucilage N 0.05% S = Nacional, 0.05 yeast, Solid mucilage N 0.0% L = Nacional, 0.0 yeast, liquid mucilage N 0.01% L = Nacional, 0.01 yeast, liquid mucilage N 0.05% L = Nacional, 0.05 yeast, liquid mucilage CCN51 0.0% S = CCN51,0.0 yeast, liquid mucilage CCN51 0.01% S = CCN51,0.01 yeast, Solid mucilage, CCN51 0.0 5% S = CCN51,0.05 yeast, Solid mucilage, CCN51 0.0% L = CCN51,0.0 yeast, liquid mucilage, CCN51 0.01% L = CCN51,0.01 yeast, liquid mucilage, CCN51 0.05% L = CCN51, 0.05 yeast, liquid mucilage.
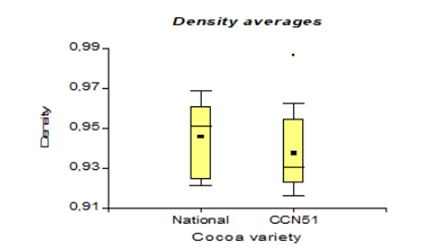
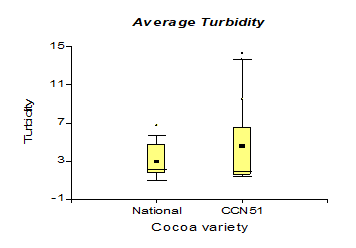
Table 3 shows the values corresponding to the density in the national cocoa variety with 0.94 g/cm3, while in CCN51, 0.93 g/cm3 were obtained. While in turbidity, the best results were 4.66 NTU, corresponding to the CCN51 variety, and in national cocoa, it was 3.07 NTU.
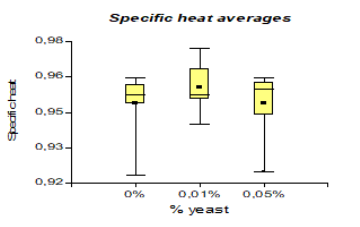
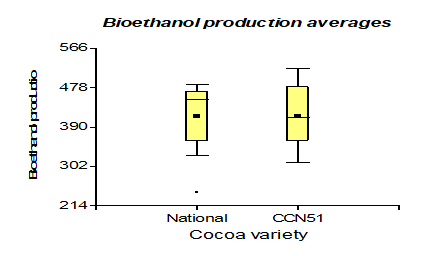
In specific heat, it presented a higher value than the national variety with 0.96 kJ/Kg °C. However, unlike cocoa CCN51 the value was 0.95 kJ/Kg °C, and to produce bioethanol, the highlight is the variety CCN51 reached a value of 414.58 mL and national cocoa 413.33 mL.
4. Results
4.1 Results discussion
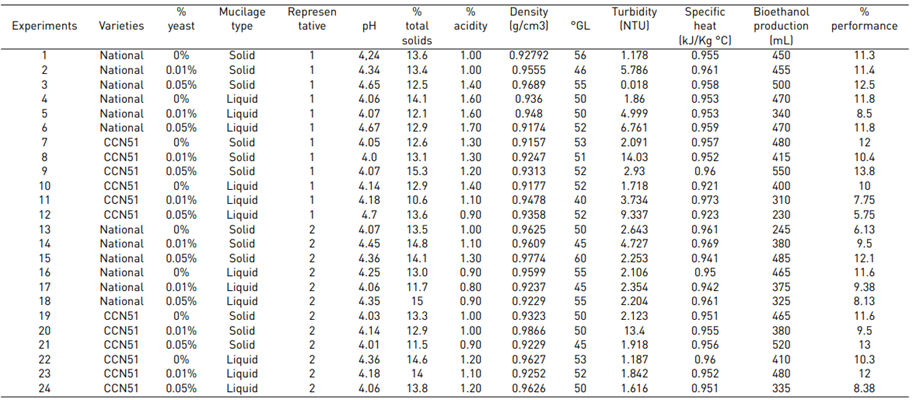
The results presented in Figure 4, regarding as for the pH, average values were obtained in the CCN51 varieties with 4.15 and Nacional with 4.35. These values are within the ranges presented in the research "Bioethanol production from lignocellulosic agro-industrial by-products" [10], with a pH of 4-7; therefore, they are similar due to the content of hydrogen ions present in cocoa mucilage. The pH, of the research above is 4.30 with the 0.01% yeast concentrations. 0% obtained a value of 4.20, these results are close to the pH (4.5-5.5) in the publication on the production of ethanol from cassava starch using the strategy of a simultaneous process of saccharification- fermentation [23], but they are lower than the data reported in the investigation of the antioxidant activity of clones of delicate and aromatic cocoa (Theobroma cacao l.) grown in the state of Chiapas-Mexico developed by González et, al. (2013) with values of 5.52-5.90 [24]. Regarding the work on optimising bioethanol production in fermentative processes of CCN51 cocoa mucilage in a batch-type bioreactor by Delgado et, al. (2018) [25], it is similar with a pH value of 4.0.
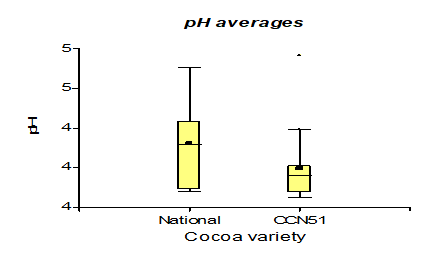
The results presented in Figure 5, regarding total solids, the cocoa mucilage variety CCN51 presented 15.05%, and the National variety obtained a value of 10.6%. In the investigation of Zapata (2010), it is found in 15% [26]. The experiments carried out with cocoa mucilage are in a higher range in the parameters proposed by Zapata (2010) due to the content of sugars present. The total solids of the cocoa mucilage have a value of 16.35, at a yeast concentration of 0.05%, while at 0%, a value of 10.5% was obtained. In the research by Pérez et al. (2013) [27], it is between 12-15%. What it indicates is close to the parameters mentioned by the studies cited. This is due to the content of sugars now present in which the yeasts break down the sugars in the cocoa mucilage.
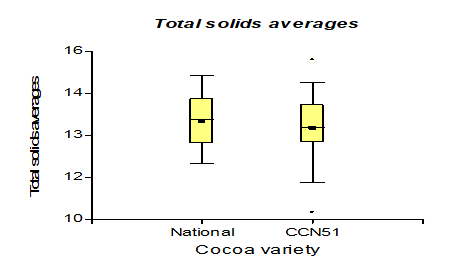
The results presented in Figure 6, regarding the acidity of the mucilage in the CCN51 varieties with a value of 0.80% and the Nacional variety with 0.90%, unlike the research [28], where their values are between 0.0-1.6%; as well as in those mentioned in [29].
They are like the acidity values of bioethanol, made from cocoa mucilage, after fermentation with different yeast percentages. Cocoa mucilage can ferment quickly and is not very acidic compared to other products, such as coffee fermentation, which is more acidic[30].
The results presented in Figure 7, regarding the alcoholic degrees presented a minimum of 50 °GL obtained in the CCN51 variety and National of 51.58 °GL. The Alcoholic Beverages Standard
determines that the °GL must be between 39-54 °GL; the procedures are within the established standards. In [17,29], there is an option to produce renewable energy from agricultural residues between 10-12 °GL. These values are lower because the cocoa mucilage has high alcoholic values due to its higher sugar content, presenting values of 47.37 °GL when working with 0.01% yeast concentrations. In comparison, 0.05% acquired a high value of 52.62 °GL, established in the NTE 368 Standard [31] .
The results presented in Figure 8, regarding bioethanol density concerning the use of varieties in CCN51 reached a value of 0.93 g/cm3 and Nacional with 0.94 g/cm3. These data are lower than the 0.991-0.995 g/cm3 indicated by Martínez (1995) [32]. The concentration of 90% alcohol reaches a density of 0.79 g/cm3 because it contains a lower concentration of alcohol, 52.62°GL and its density changes. While, in the investigation of [25], it is 1.05 g/cm3. This value is higher because the raw material (banana) is denser than the cocoa mucilage. The density in relation to the yeast concentrations at 0.01% with 0.95 g/cm3, and at 0% reached a value of 0.93 g/cm3.
These values are lower than the 1.11-1.07 g/cm3 suggested by [33]. There is a difference due to the relationship between the mass and the volume of bioethanol due to the effect of the yeast percentage in the fermentation process.
The results presented in Figure 9, regarding the analyses obtained from the turbidity taken from bioethanol present a value of 4.66 NTU corresponding to the CCN51 variety and a minimum value in the Nacional variety with 3.07 NTU. These data are like those obtained by Díaz et al. (2014) [34] (3.07-4.66 NTU), which leads us to understand that cocoa mucilage can be a natural coagulant to reduce the initial turbidity in fermented liquids (wine, beer). The turbidity increased to a value of 6.35 NTU, corresponding to the experiment with a yeast concentration of 0.01%, and when the yeast was used at 0%, 1.86 NTU of turbidity was obtained. When working with cocoa mucilage in solid form, turbidity acquired a value greater than 4.42 NTU, and cocoa mucilage in liquid form with a value of 3.30 NTU. These results are similar to those reported by Cerón (2013) (3.40-4.75 NTU) [35] in research on the environmental characterization of sugarcane residues still resulting from ethanol production, due to the transparency provided by ethanol from cane sugar, it has a better appearance.
The results presented in Figure 10, regarding the specific heat, it presented a value of 3.99 kJ/Kg °C, which corresponds to National variety, while in CCN51 3.98 kJ/Kg °C was obtained. In the research carried out by Escobar et al. (2008) [36] establishes that the specific heat must be 2.38 kJ/Kg °C. The specific heat presented a value greater than 0.96 kJ/Kg °C, which corresponds to 0% yeast concentration, while at a concentration of 0.01%, 0.94 kJ/Kg °C was obtained. Regarding the mucilage type, 0.96 kJ/Kg °C is obtained, which corresponds to solid mucilage, while in liquid mucilage, 0.95 kJ/Kg °C is obtained. Comparing with the research data of Castillo (2012) and Castillo (2010) [22,37], it establishes that the results should be between (0.68-0.92 kJ/Kg °C). This supports the investigation that experiments are within established ranges.
The results presented in Figure 11, regarding bioethanol production, values of 414.58 mL of cocoa mucilage of the CCN51 variety were obtained and 413.33 mL for the Nacional variety. These values are in the range of 77.52-572.64 mL, mentioned by Betancourt (2009) [38] on general perspectives for the development of the biofuel industry in Uruguay. The yield of the final product obtained from sugar cane or cocoa is similar to the sample's weight. In bioethanol production, the most representative value is when the application of yeast (at 0.05%) gives a value of 426.87 mL, and at 0.01%, a value of 391.87 mL is obtained. These data for 0.05% yeast are related to those mentioned in the study on bioethanol production from mixtures of sugar cane molasses juice [39] (405.2-551.4 mL). Bioethanol production in the mucilage type has values of 443.75 mL in solid and 384.16 mL in liquid.
5. Conclusions
Cocoa mucilage can be used as an alternative to producing bioethanol. The data obtained according to the cocoa variety CCN51, showed a significant difference in the physicochemical characteristics. A pH of 4.07, 12.9% total solids, 0.9% total acidity, 60 alcoholic degrees, turbidity of 2.93, the density of 0.91 g/cm3 and specific heat of 0.92 kJ/Kg °C were obtained. To increase its purity, a second distillation must be carried out, reaching up to 80 alcoholic degrees.
The conditions to produce bioethanol from cocoa mucilage were optimised using different concentrations of yeast. Saccharomyces cerevisiae yeast percentages were 0%, 0.01%, and 0.05%. The most suitable percentage of yeast for the cocoa mucilage fermentation process is 0.05%. This allows consuming sugars easily and generating more alcoholic content, giving bioethanol values of 426.87 mL. One of the key points in the experimental stage is fermentation, which must occur in a controlled medium (pH between 4-6); if it is too acidic, it causes the yeast to die, resulting in incomplete fermentation.
This research shows positive results in bioethanol production from cocoa mucilage under controlled conditions (laboratory) with anaerobic fermentation. The cocoa mucilage has a high potential for use as a substrate in fermentative processes to produce alcohol, the same that reaches a production with a more representative value when applying yeast at 0.05%, giving a value of 426.87 mL of bioethanol. Furthermore, it is shown that there is a significant difference between bioethanol production due to the mucilage type (solid or liquid) since in solid, there is a more significant number of sugars that are transformed into ethanol.