Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
CES Medicina
versión impresa ISSN 0120-8705
CES Med. v.24 n.2 Medellín jul./dic. 2010
ARTÍCULOS DE REVISIÓN
Understanding medical treatment in lower limb peripheral arterial disease
Entendiendo el manejo médico de la enfermedad arterial periférica de los miembros inferiores
HEINZ HILLER,1; NATALIA MURILLO2; JORGE HERNANDO ULLOA2; JORGE ULLOA DOMINGUEZ3
1 Vascular Surgery Fundación Cardioinfantil Bogotá Colombia correo electrónico: heinzhiller@gmail.com
2 Vascular Surgery Clínica de Venas de Colombia
3 Vascular Surgery Fundación Vascular de Colombia
ABSTRACT
The management of patients with peripheral arterial disease has changed dramatically over the last ten years. Surgery, once the preferred treatment method for intermittent claudication, is not longer the treatment of choice for patients with this presentation. Medical management is now the first choice, and an improvement of claudication and quality of health can be achieved using a combination of statins, platelet aggregation inhibitors and the new inhibitor of cyclic AMP (adenosin monophosfate), Cilostazol. This review focuses on the new recommendations for clinical evaluation, diagnosis and medical treatment of patients with the disease.
KEY WORDS
Peripheral Vascular Diseases, Cilostazol, Intermitent claudication, Platelet aggregation inhibitors, Ankle Brachial Index
RESUMEN
El manejo de pacientes con enfermedad arterial periférica ha cambiado dramáticamente en los últimos diez años. La cirugía para el tratamiento de la claudicación intermitente, que en años anteriores fue el tratamiento de elección, ha sido reemplazada como primera opción por el tratamiento médico. En la actualidad se puede lograr una mejoría en la claudicación y la calidad de vida con el uso de estatinas, inhibidores de la agregación plaquetaria y el nuevo inhibidor de la AMP cíclica (adenosin monofosfato), Cilostazol. Esta revisión se enfoca en las recomendaciones actuales para la evaluación clínica, la aproximación diagnóstica y el tratamiento médico de pacientes con la enfermedad.
PALABRAS CLAVE
Enfermedad arterial oclusiva periférica, Cilostazol, Claudicación intermitente, Inhibidores de la agregación plaquetaria, índice tobillo brazo
INTRODUCTION
Vascular surgery is unique amongst surgical specialties, because it includes not only surgical treatment options but also non-invasive medical therapies for patients with peripheral arterial disease (PAD). This wide spectrum of alternatives results in patients with PAD not being detected early while management options remain widely unknown to general practitioners (1). The recent ATTEST (prise en charge de l'ArTériopaThie oblitErante des membreS inférieurs chez les paTients en médecine générale) study in France revealed a serious lack of adequate management of patients with PAD. It showed that only 13% of patients received standard quadruple therapy with angiotensin converting enzyme inhibitors, statins, and platelet aggregation inhibitors while the risk of amputation was overestimated by more than 56% of physicians (2). Even further, in a study by McDermott and associates in 2000, only 20% of patients have been told that they have PAD (3).
As the life expectancy increases due to advances in medicine, vascular diseases have become increasingly frequent, creating the need to diagnose and treat PAD in early stages. The prevalence of PAD increases with age, and is prevalent in up to 20% of non diabetic patients older than 70 years (4-5). The spectrum of the disease can range from asymptomatic patients (which constitute the majority of patients), patients with intermittent claudication and patients with rest pain and critical ischemia. In diabetic patients the prevalence of PAD increases to 45% as well as the amputation rates. Modern medical and interventional management have reduced amputation numbers, making it possible for non diabetic patients with intermittent claudication to have a lifelong risk of less than 2% mayor limb amputation (6). Thus, modern medical management has proven to be superior than elective surgery and bypass reconstruction for the improvement in walking distance in patients with intermittent claudication.(7)
PAD is a marker of early mortality due to cardiovascular events. 5 and 7 year mortality rates in patients with rest pain can be as high as 60% and 70%, respectively (8). Declining ankle brachial index is directly proportional to the risk of coronary events (9). Multiple risk factors have been associated with these high mortality rates, such as tobacco use, advanced age, dialysis dependence and diabetes. In one of the classical studies published in 1981 by Hertzer et al, the research group demonstrated a prevalence of 90% of angiografically detectable coronary disease in patients undergoing a peripheral vascular procedure (10).
EVALUATION AND CLASSIFICATION OF PERIPHERAL VASCULAR DISEASE
Clinical classification
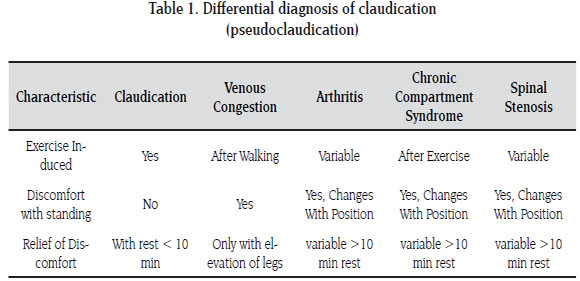
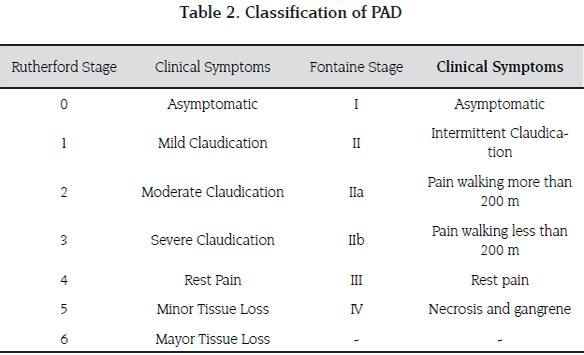
Because of the traditional age group in which chronic limb ischemia presents, the role of the vascular surgeon is aimed at identifying patients with arterial insufficiency and differentiating it from other causes of limb pain such as degenerative arthritis, radiculopathy and other osteo -muscular diseases (11) (Table 1). Chronic limb ischemia has a spectrum of clinical manifestations that range from asymptomatic to severe critical limb ischemia. Symptoms only occur when the demand for oxygen is higher than the blood supply in the presence of stenotic lesions of peripheral arteries. In sedentary patients symptoms may therefore not be apparent initially unless a stress test is applied. There are two main classification systems: Fontaine and Rutherford, the former is widely adopted and used in most publications. Fontaine is based on clinical symptoms and signs rather than diagnostical findings. Patients with Fontaine Stage I are asymptomatic, II; claudication, III; rest pain, and IV present with necrosis or ulceration (Table 2) (12).
This classification can vary, however, because a subset of patients who are initially classified in stage I, have pain only with specific forms of exercise or only induced with extreme exercise as mentioned earlier. Careful clinical evaluation is therefore essential. Functional assessment of the degree of arterial compromise can be achieved by asking the patient to describe the nature of the symptoms, at what distance (usually measured in meters) they occur, if the pain subsides after walking and if it's present at rest or forces him to leave his bed at nighttime. It is very important to ask how long the pain persists after the patient has stopped walking; typical claudicants require less than 10 minutes of rest to resume activities. Longer resting times are more closely related to other causes of pain with exercise (termed pseudoclaudication) such as radiculopathy and osteoarthrosis (13).
It can be very challenging for the specialist to identify other causes of leg pain, especially those caused by nerve entrapment by a herniated disc or ostephyte at the lumbar root level, as this too can cause pain on walking and standing, or at night. A systematic review of the significance of clinical signs was published in 2006 by Khan et al (14).17 articles related to accuracy and precision of clinical signs where included. The most reliable clinical sign to predict the presence of PAD was the absence or presence of palpable pulses. The likelihood of having PAD was further increased in patients with intermittent claudication, and with additional risk factors such as age, smoking, dyslipidemia and diabetes mellitus. Other useful clinical sings such as skin changes, bruits and Buerger´s test were evaluated. When present, these signs were useful in predicting the presence of PAD, but their absence, especially in patients with other risk factors, were not useful to exclude PAD. No other clinical sign was useful in lowering the risk for a patient having PAD. Capillary refill time had a very poor diagnostic accuracy in this study. The individual likelihood ratios have to be used simultaneously with the pretest probabilities (e.g. the risk factors) as none of the individual signs can rule out PAD completely. However, when the combination of normal clinical signs and low risk factors are present, the physician can safely rule out PAD. It is imperative to gather additional information regarding other vascular beds (coronary and cerebral) and other risk factors for atherosclerosis. This information is valuable for current treatment and for prevention of further complications following therapy. (15).
Ankle Brachial Pressure Index
The ankle brachial pressure index (ABPI) is one of the most useful tools to evaluate the state and prognosis of patients with PAD due to its high sensitivity and specificity (16). Other methods are available, such as duplex scanning, arteriography and CT-angiography, but the ABPI is non invasive, readily available and can be applied and interpreted by any trained person. It has been validated in multiple clinical and epidemiological studies, proving its reliability in classifying the severity of PAD and helping physicians and vascular surgeons to make decisions on adequate management options. It is also very useful in determining the outcomes of any therapeutical approach (17).
A very recent study on the correlation of symptom severity and ABPI was published by Mc Dermott and colleagues. They found a direct connection between ABPI and walking distance on a 6 minute treadmill test. With an ABPI of 0.7-0.9 the average distance was 355 meters, with 0.5-0.7, 316 meters and with an ABPI of less than 0.5 the distance was 286 meters (18). The same effect was also observed when analyzing the amount of time spent on the treadmill which was shorter for lower ABPIs.
The method for calculating the index is the ratio of the highest ankle systolic pressure divided by the highest brachial systolic pressure in each side (19). As a general recommendation by most vascular laboratories, the preferred method for auscultation the pulses after deflation of the pressure cuff is using a hand-held Doppler with frequencies of 8 mHz and this should be used for every measurement. With the patient lying supine, the pressure cuff of the sphygmomanometer is placed two finger-breadths above the pulse. The cuff is inflated above the highest systolic pressure, then measured and deflated at a rate of 3mmHg per second. The pressure at which the doppler sign reappears is recorded. The systolic pressure should be measured at the brachial, pedal and posterior tibialis arteries.
An abnormal result is considered when the ABPI is < 0.9, with a sensitivity of 95% and specificity close to 100% compared to angiography (20). Mild PAD is indicated at ratio values of 0.71 to 0.9, moderate PAD is at 0.41 to 0.7 and severe PAD is represented by values less than 0.4 (21). Rest pain starts to appear at values of 0.5 and gangrene and necrosis at values of 0.2 or less. Careful evaluations of falsely high values is imperative, as ratios of 1.2 or higher are associated to calcification, especially in diabetic patients, caused by the inability of the pressure cuff to occlude the artery adequately.
Additional diagnostic modalities
Once clinical classification is made, the next step is identifying the relevant anatomical segments where the stenotic lesions are using radiological tests. Bi-dimensional and color imaging of peripheral arteries is a very sensitive and specific diagnostic tool that can help the treating physician to understand the disease and evaluate the state of collateral blood flow. The report of a correctly performed duplex scan of lower limb arteries should include the degree of calcification, percentage of stenosis, flow velocities distal to the stenosis and identification of post occlusive blood flow due to collateral flow amongst others (22-23).
Other diagnostic methods such as arteriography, magnetic resonance angiography and CT angiography are usually reserved to make decisions on interventional or surgical procedures, and not for making diagnosis of the disease, as these modalities are prone to complications (related to puncture, contrast and irradiation) (24).
MANAGEMENT OF RISK FACTORS
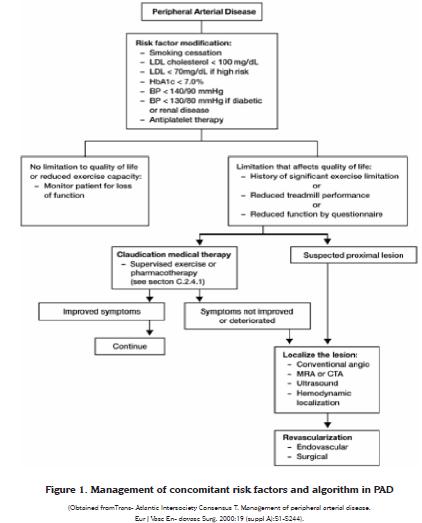
The American Heart Association and the American College of Cardiology have issued in 2001 general recommendations for the management of modifiable risk factors in patients with vascular disease, especially for smoking, dyslipidemia, hypertension, obesity and diabetes (25). This section will focus mainly on the effects of smoking cessation on PAD and the effects of the use of statins in these patients. A summary of the management algorithm is depicted in figure 1.
Smoking and PAD
Very interestingly, the majority of patients are still unaware of the effects of smoking on PAD. Many have still the concept that smoking only causes emphysema or lung cancer. Cessation of smoking is the most important modifiable risk factor in PAD in observed studies. Burns and colleagues have shown in a study published in 2003 that patients who stop smoking reduce their mortality 36 Revista CES MEDICINA Volumen 24 No.2 Julio - Diciembre / 2010 rate from 54% to 18% in a ten year period. Additionally smoking was associated to progression of the disease up to having rest pain (Fontaine III), compared to patients who quit in a seven year period (26). It is therefore very important to identify patients who currently smoke and offer advice and therapy to assist smoking cessation. Several therapies have been suggested by a recent guideline form the U.S. Public Health Service, such as the use of nicotine patches, inhalers, gum or bupropion (27). Information on prescription and use of these medications is beyond the scope of this article.
Statins and improvement of quality of life
More than 40 years ago, the association between high cholesterol levels and cardiovascular disease was established (28). Reducing cholesterol level improves mortality risk from cardiac and vascular diseases as it was first established in the ancillary 4-S trial with simvastatin in 1994 (29). Since then, several studies have been published of the effect of lipid lowering agents, and specifically statins, on PAD. In two different studies published in 2003, the effect of statins vs. placebo on walking distance at 6 and 12 months was studied. Aronow et al demonstrated an improvement of the walking distance by 24% and 42% at 6 and 9 months respectively. The placebo had no effect whatsoever on improvement of walking distance (30). Similarly, in the Italian study, additional to walking distance, ABPI and quality of life was measured with or without simvastatin treatment. Patients taking 40 mg a day of simvastatin had an increase of walking distance of more than 90 meters at 6 months, an improvement of more than 0.2 in the ABPI and an improvement in the quality of claudication symptoms (31).
It seems that not only are there beneficial effects of statins on cholesterol levels, but also on the functional capacity of patients with PAD and the rate of decline in functional status over time. This has been consistently demonstrated in additional trials using walking distance and quality of life as measures of the effect of statins on PAD (32). In addition to these findings, in more recent studies including the media-intimae thickness of carotid arteries, statins may have an overall reducing effect on the thickness of plaques, a direct effect on the endothelial dysfunction in patients with PAD. At present, there are several studies addressing this issue. The current recommendation according to the Intersociety Consensus for the Management of PAD (TASC II) for all patients with PAD is to take a statin once a day even in the presence of normal cholesterol levels (33).
Exercise and resistance training
Claudication pain is caused mainly due to the excessive production of lactate from anaerobic glicolysis in patients with decreased supply of oxygen in PAD. Muscles have to adapt to the chronic lack of blood, and hence oxygen supply, to improve the use of glycogen stores without producing lactate (34). It is by means of this mechanism that a subgroup of patients, who slowly develop stenotic lesions over time, become more adept at using this limited blood supply and can be asymptomatic at the time of consultation, even in the absence of palpable distal pulses. Initially increased collateral blood flow was thought to be the cause of this adaptation, but a study by Jansson et al, revealed the adaptations of muscles to be the principal mechanism for improving claudication (35).
More than 10 randomized controlled studies have recently proven that increasing muscle exercise increases muscle adaptation and thus improvement of symptoms. Even in patients without specific training, higher daily physical activity reduces de functional decline of walking distance and intermittent claudication (36). One of the earliest trials by Larsen et al in 1966 already showed a beneficial effect of regular exercise on walking distance in patients with PAD (37). A Cochrane systematic review published in 2000, again proved convincing, in a summary of 10 trials that included a supervised treadmill exercise protocol, that the therapy significantly improved maximal walking time by a mean difference over 6 minutes, with an overall improvement in walking ability of approximately 150% (range 74% to 230%) (38).
A more recent randomized controlled trial published in 2009, compared resistance training vs. treadmill training and the effect on intermittent claudication. Both methods improved significantly walking distance and quality of life of patients, whilst resistance training had a better effect on overall functional performance measured by walking distance, quality of life questionnaire and step climbing ability (39). The TASC II (19) recommends supervised treadmill exercise training for all eligible patients with PAD (grade A recommendation). It also states that the most beneficial protocol consists of a treadmill or walking that is of sufficient intensity to bring on the claudication followed by rest and resuming the walking for a 60 minute session, to be conducted at least 3 times a week.
Platelet aggregation inhibitors
Aspirin or acetylsalicylic acid is widely used for prevention of ischemic events in patients with cardiovascular disease. This was proven in the key work by the Antithrombotic Trialists' Collaboration in 1994 (40) and subsequently ratified in 2002 with a Cochrane systematic review (41) showing an overall reduction in cardiovascular events and death of 24% using a low dose treatment (75-100 mg of aspirin) once a day. This study applied mostly to reduction in coronary and cerebral vascular events and no specific mention of ischemic risk reduction in PAD patients was mentioned. To date, the treatment is clearly indicated in PAD patients, mainly because of the increased association with coronary and cerebral disease (more than 60% of patients with PAD have atherosclerosis in other organs), and therefore, lower limb ischemic risk reduction is only proven in patients with coexisting disease.
Several studies attempting to address this issue have been published recently. The CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events study), assessed the reduction effect on overall ischemic events using either aspirin or clopidrogel (42). A subgroup analysis revealed an overall relative risk reduction of 24% of cardiovascular ischemic events in patients with PAD. Clopidrogel was equally safe to aspirin in regards to thrombocytopenia. Nevertheless, patients with platelet aggregation inhibitor treatment have an increased risk of bleeding and temporary suspension might be required previous to surgery or other endovascular procedures. Subsequently, several trials compared the efficacy of aspirin versus other platelet aggregation inhibitor agents (such as ticlopidine and other thienopyridines), with no statistical difference on event risk reduction or complication rates(43). The CAPRIE study also showed that combination therapy had no additive effects on the risk reduction of events in PAD patients. At present, there are no studies to show any improvement in management with combination therapy.
The Critical Leg Ischemia Prevention Study Group (CLIPS) is the first to conduct a multi-center, randomized controlled trial on the prophylactic effect of platelet aggregation inhibitor agents, focusing exclusively on patients with PAD (44). The study involved thirty seven centers across Europe, recruiting 366 outpatients with PAD stage I-II, and ABPI less than 0.8. In 210 patients, who completed follow-up over a period of 2 years, there was a statistically significant reduction of peripheral vascular events by 26%. This was the first study to show direct evidence of the benefit of low dose aspirin in this context and current recommendations include the use of a low dose platelet aggregation inhibitor agent, but not combination therapy.
Cilostazol
Cilostazol was approved by the FDA in 1999 for use in intermittent claudication. Cilostazol is an inhibitor of phosfodiesterase III, increasing cyclic adenosine monophosfate and subsequently vasodilation form smooth muscle cell relaxation. It also has antiplatelet effects, and potentially beneficial effects on plasma lipoprotein levels. The exact mechanisms by which it improves claudication distance are still unknown and additional effects may explain them (45).
A metaanalysis proving the benefits of Cilostazol by showing an increase in walking distances and quality of life was published in 2002 by Thompson et al. It analyzed 8 randomized controlled trials evaluating the effect of Cilostazol on quality of life including the use of SF-36 questionnaires (46). A total of 2702 patients were included from all studies, and the treatment period ranged from 12 to 24 weeks. Cilostazol increased walking distance by 50% and 67% respectively, as well as the scores on quality of life. Although patients taking Cilostazol had more side effects, no serious adverse effects were reported. Some benefits on lipoprotein levels included a decrease of triglyceride levels by 15.8% and an increase in high density lipoprotein levels by 12.8%.
Cochrane published a systematic review of the literature in 2007 (47). The authors found similar results comparing all randomized trials included. In addition, Cilostazol was not associated with any mayor adverse events including cardiovascular events or mortality in patients receiving Cilostazol compared with placebo. Thus the recommendation to date is to use Cilostazol in the primary management of intermittent claudication.
Other therapeutic options
Most additional therapies are aimed at causing vasodilatation to improve oxygen delivery via the collateral blood flow system. The most common drugs used in this setting in addition to Cilostazol, are the Prostaglandin E (PGE1 and PGE2) analogues. Traditionally, these therapies have been used in patients with inoperable disease and when all other medical treatment options have been exhausted. Currently these indications are being revisited as a result of a recent metaanalysis published in 2005 by Amendt (48). The most recent indication is to use PGE1 in patients who are unable to tolerate exercise training. The author included 13 studies with different analogues of PGE11 (Iloprost, Beraprost and AS-013). He found an increase in maximal pain free distance walking and maximal walking distance by more than 107% compared to other PGE and placebo with minimal side effects compared to other substances.
Stem cell therapy and endothelial therapy have shown promising results in treating intermittent claudication and critical ischemia, but their discussion is beyond the scope of this article and subject for another review.
DISCUSSION
Current management of peripheral vascular disease and intermittent claudication has changed dramatically in the recent years with the advent of newer and effective therapeutic options. Surgery is no longer a treatment indication for claudication as proven by the trials discussed in previous paragraphs as patency rates are inadequate after 3 to 5 years post intervention. Endovascular treatment has gained popularity as it is minimally invasive and can be performed using local anesthesia. A recent survey by Taylor et al. reviewed retrospectively 1000 treated limbs for intermittent claudication at the Greenville Academic Department of Surgery (49). Two thirds of patients were treated with endovascular techniques and around 96% of patients were able to maintain ambulatory independence at 5 years. Endovascular therapy was slightly superior to surgery in the long term.
The multimodal medical management, using statins, vasodilator agents, platelet aggregation inhibitor therapy and exercise training is not only limited to patients with concurrent disease. Risk factor modification is essential, including quitting smoking, strict control of hypertension and glucose levels. The use of statins is indicated even in the absence of dyslipidemia. The most recent systematic review and metaanalysis of the effectiveness of medical therapy for intermittent claudication was published in the European Journal of Vascular and Endovascular Therapy by Momsen et al. More than 43 trials that met the inclusion criteria where included in this analysis (50). Vasodilator agents showed a moderate improvement in maximal walking distance, but the greatest benefit seems to come from the use of statins. The recommendation thus stands on the use of statins at adequate dosages.
It is imperative to establish a correct diagnosis of the disease, trying to identify causes of pseudoclaudication. Once a correct diagnosis is made, staging is useful to establish a baseline for treatment comparison in the future and to document any improvement in the scale (Table 2). This assessment must include the ABI.
In patients with intermittent claudication and no rest pain, treatment can be initiated with no further imaging studies. If these conditions are not met, referral to a vascular centre becomes necessary. Duplex scan of affected arteries can be obtained if more proximal lesions are suspected and/or treatment fails to improve walking distance within a 6 month period. The use of antiplatelet agents, Cilostazol and statins should be liberal, unless contraindicated. Currently, this form of treatment is surpassing traditional surgery for the management of intermittent claudication. The indication to intervene a patient surgically has changed inversely to the former indications for medical therapy. As the multimodal disease process is more widely understood, newer drugs become available to treat and possibly prevent the formation of atheromas in patients at risk and will eventually decrease amputation rates worldwide.
BIBLIOGRAPHY
1. Hirsch AT, Hiatt WR. PAD awareness, risk, and treatment: New resources for survivalâ the USA PARTNERS program. Vasc Med 2001;6:9-12. [ Links ]
2. Blacher J, Cacoub P, Luizy F, Mourad JJ, Levesque H, Benelbaz J, et al. Peripheral arterial disease versus other localizations of vascular disease: the ATTEST study. J Vasc Surg 2006;44(2):314-8. [ Links ]
3. McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic PAD is independently associated with impaired lower extremity functioning: The women's health and aging study. Circulation 2000;101:1007-12. [ Links ]
4. Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation 1985;71:510-15. [ Links ]
5. Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. J Am Med Assoc 2001;286:1317-24. [ Links ]
6. Dormandy JA, Murray GD. The fate of the claudicant: a prospective study of 1969 claudicants. Eur J Vasc Surg 1991;5:131-3. [ Links ]
7. Wilson S, Gelfand D, Jimenez J, Gordon I. Comparison of the results of percutaneous transluminal angioplasty and stenting with medical treatment for claudicants who have superficial femoral artery occlusive disease. Vascular 2006;14(2):81-7. [ Links ]
8. Criqui MH. Peripheral arterial disease and subsequent cardiovascular mortality: A strong and consistent association. Circulation 1990;82:2246-7. [ Links ]
9. Weatherley BD, Nelson JJ, Heiss G, Chambless LE, Sharrett AR, Nieto FJ, et al. The association of the ankle-brachial index with incident coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study, 1987-2001. BMC Cardiovasc Disord 2007;16:7-13. [ Links ]
10. Hertzer NR. Fatal myocardial infarction following lower extremity revascularization: Two hundred seventy three patients followed six to eleven postoperative years. Ann Surg 1981;193:192-8. [ Links ]
11. Cronenwett JL, Rutherford RB. Decision making in vascular surgery. Philadelphia,: WB Saunders; 2001. [ Links ]
12. Fontaine R, Kim M, Kieny R. Die chirugische Behandlung der peripheren Durchblutungsstörungen. (Surgical treatment of peripheral circulation disorders) (in German). Helvetica Chirurgica 1954;21:499-533. [ Links ]
13. Welch HJ. Chronic lower extremity ischemia. Compr Ther 1997;23:534-38. [ Links ]
14. Khan NA, Rahim SA, Anand SS, Simel DL, Panju A. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295(5):536-46. [ Links ]
15. Stoffers HE, Kester AD, Kaiser V. The diagnostic value of signs and symptoms associated with peripheral arterial occlusive disease in general practice: A multivariate approach. Med Decis Making 1997;17:61-70. [ Links ]
16. Doobay AV, Anand SS. Sensitivity and specific- ity of the ankle-brachial index to predict future car- diovascular outcomes: a systematic review. Arterio- scler Thromb Vasc Biol 2005;25:1463-9. [ Links ]
17. Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, et al. Cardiovascular Health Study (CHS) Collaborative Re- search Group. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Circulation 1993;88:837-45. [ Links ]
18. McDermott MM, Ferrucci L, Guralnik JM, Dyer AR, Liu K, Pearce WH, et al. The ankle brachial index is associated with the magnitude of impaired walking endurance among men and women with peripheral arterial disease. Vasc Med 2010;[Epub ahead of print] (may 28). [ Links ]
19. Trans- atlantic Intersociety Consensus T. Management of peripheral arterial disease. Eur J Vasc En- dovasc Surg. 2000;19 (suppl A):S1-S244. [ Links ]
20. Baxter GM, Polak JF. Lower limb colour flow im- aging: a comparison with ankle:brachial measurements and angiography. Clin Radiol. 1993;47:91-5. [ Links ]
21. Sumner DS, Strandness DEJ. The relationship be- tween calf blood flow and ankle blood pressure in patients with intermittent claudication. Surgery. 1969;65:763-71. [ Links ]
22. Wilson YG, George JK, Wilkins DC, Ashley S. Duplex assessment of run-off before femorocrural reconstruction. Br J Surg. 1997;84:1360-3. [ Links ]
23. Wain RA, Berdejo GL, Delvalle WN. Can duplex scan arterial mapping replace contrast arteriography as the test of choice before infrainguinal revascularization? J Vasc Surg 1999;29:100-7. [ Links ]
24. Grassbaugh JA, Nelson PR, Rzucidlo EM, Schermerhorn ML, Fillinger MF, Powell RJ, et al. Blinded comparison of preoperative duplex ultrasound scanning and contrast arteriography for planning revascularization at the level of the tibia. J Vasc Surg. 2003;37:1186-90. [ Links ]
25. Smith SJ, Blair S, Bonow R, Brass LM, Cerqueira MD, Dracup K, et al. AHA/ACC Guidelines for Preventing Heart Attack and Death in Patients with Atherosclerotic Cardiovascular Disease: 2001 update. A statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 2001;104:1577-9. [ Links ]
26. Burns P, Gough S, Bradbury AW. Management of peripheral arterial disease in primary care. BMJ USA 2003;3:277-81. [ Links ]
27. The tobacco use and dependence clinical practice guideline Panel S, and Consortium Representatives. A clinical practice guideline for treating tobacco use and dependence: A US Public Health Service report. JAMA. 2000;283:3244-54. [ Links ]
28. Murabito JM, D' Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication: A risk profile from the Framingham Heart Study. Circulation 1997;96:44-9. [ Links ]
29. Scandinavian, Simvastatin, Survival, Study, Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344(8934):1383-9. [ Links ]
30. Aronow WS, Nayak D, Woodworth S, Ahn C. Effect of simvastatin versus placebo on treadmill exercise time until the onset of intermittent claudication in older patients with peripheral arterial disease at six months and at one year after treatment. Am J Cardiol. 2003;92(6):711-2. [ Links ]
31. Mondillo S, Ballo P, Barbati R, Guerrini F, Ammaturo T, Agricola E, et al. Effects of simvastatin on walking performance and symptoms of intermittent claudication in hypercholesterolemic patients with peripheral vascular disease. Am J Med 2003;114(5):359-64. [ Links ]
32. McDermott MM, Guralnik JM, Greenland P, Pearce WH, Criqui MH, Liu K, et al. Statin use and leg functioning in patients with and without lower-extremity peripheral arterial disease. Circulation 2003;107(5):757-61. [ Links ]
33. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Int Angiol. 2007;26(2):81-157. [ Links ]
34. Alexander RW, Dzau VJ. Vascular biology: the past 50 years. Circulation 2000;102 (Suppl 4):IV112-IV6. [ Links ]
35. Jansson E, Johansson J, Sylvén C, Kaijser L. Calf muscle adaptation in intermittent claudication. Side-differences in muscle metabolic characteristics in patients with unilateral arterial disease. Clinical Physiology 1988;8(1):17-29. [ Links ]
36. Garg PK, Liu K, Tian L, Guralnik JM, Ferrucci L, Criqui MH, et al. Physical activity during daily life and functional decline in peripheral arterial disease. Circulation 2009;119(2):251- 60. [ Links ]
37. Larsen OA, Lassen NA. Effect of daily muscular exercise in patients with intermittent claudication. Lancet 1966;2(7473):1093-6. [ Links ]
38. Leng GC, Fowler B, Ernst E. Exercise for intermittent claudication (Review). Cochrane Database of Systematic Reviews 2000(2):Art. No.: CD000990. [ Links ]
39. McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA 2009;301(2):1665-74. [ Links ]
40. ATC. (Antiplatelet Trialists Colaboration), Collaborative overview of randomised trials of antiplatelet therapy-I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Br Med J 1994;308(6921):81-106. [ Links ]
41. Reiter M, Bucek RA, StÃmpflen A, Minar E. Prostanoids for intermittent claudication. Cochrane Database Syst Rev. 2004;1:CD000986. [ Links ]
42. Bhatt D, Fox K, Hacke W, Berger P, Black H, Boden W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706-17. [ Links ]
43. Hiatt WR, Krantz MJ. Masterclass series in peripheral arterial disease. Antiplatelet therapy for peripheral arterial disease and claudication. Vasc Med 2006;11(1):55-60. [ Links ]
44. CLIPS, Catalano M, Born G, Peto R. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. Critical Leg Ischaemia Prevention Study (CLIPS) Group. J Intern Med 2007;261(3):276-84. [ Links ]
45. Kohda N, Tani T, Nakayama S, Adachi T, Marukawa K, Ito R, et al. Effect of cilostazol, a phosphodiesterase III inhibitor, on experimental thrombosis in the porcine carotid artery. Thromb Res 1999;96:261-68. [ Links ]
46. Thompson PD, Zimet R, Forbes WP, Zhang P. Meta-analysis of results from eight randomized, placebo-controlled trials on the effect of cilostazol on patients with intermittent claudication. Am J Cardiol 2002;90:1314-9. [ Links ]
47. Robless P, Mikhailidis DP, Stansby GP. Cilostazol for peripheral arterial disease. Cochrane Database Syst Rev. 2007;1:CD003748. [ Links ]
48. Amendt K. PGE1 and other prostaglandins in the treatment of intermittent claudication: a meta-analysis. Angiology. 2005;56(4):409- 15. [ Links ]
49. Taylor SM, Kalbaugh CA, Healy MG, Cass AL, Gray BH, Langan EM, et al. Do current outcomes justify more liberal use of revascularization for vasculogenic claudication? A single center experience of 1.000 consecutively treated limbs. J Am Coll Surg 2008;206(5):1053-62. [ Links ]
50. Momsen AH, Jensen MB, Norager CB, Madsen MR, Vestersgaard-Andersen T, Lindholt JS. Drug therapy for improving walking distance in intermittent claudication: a systematic review and meta-analysis of robust randomised controlled studies. Eur J Vasc Endovasc Surg. 2009;38(4):463-74. [ Links ]
Recibido en: sept 6 de 2010; revisado en: octubre de 2010; aceptado en: noviembre 20 de 2010
Forma de citar: Hiller H, Murillo N, Ulloa JH, Ulloa-Domínguez J. Understanding medical treatment in lower limb peripheral arterial disease. Rev CES Med 2010;24(2):LLLLLL