Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
CES Medicina
versión impresa ISSN 0120-8705
CES Med. vol.27 no.1 Medellín ene./jun. 2013
Prevalence and antimicrobial susceptibility of Staphylococcus aureus methicilin resistant isolated from medical students
Prevalencia y susceptibilidad antimicrobiana de cepas de Staphylococcus aureus meticilino resistentes aisladas de estudiantes de Medicina
IVÁN ALBERTO MÉNDEZ1, DIEGO FABIÁN HOLGUÍN-RIAÑO2, DIANA PATRICIA PACHÓN-BARINAS1,
FRANCISCO JAVIER AFRICANO2, IVÁN MAURICIO GONZÁLEZ2, NYDIA ALEXANDRA ROJAS3
1 Docente enfermedades infecciosas, Facultad de Medicina, Universidad Militar, Bogotá D.C.
2 Estudiante Facultad de Medicina, Universidad Militar, Bogotá D.C.
3 Directora de Programa, Universidad Militar, Bogotá D.C.,
Recibido: noviembre 26 de 2012. Revisado: febrero 11 de 2013. Aceptado: marzo 15 de 2013
ABSTRACT
Objective: To establish the prevalence and identify the level of resistance to methicillin, vancomycin and alternative antibiotics in Staphylococcus aureus isolates from medical students in clinical training.
Materials and methods: A cross-sectional observational design with non-random sampling was used in medical students during clinical training in a tertiary healthcare facility. Samples were taken from nasal and hands swabs and cultured on blood agar. For beta-hemolytic gram-positive cocci, catalase and coagulase tests were performed and then cultured on mannitol salt agar. Susceptibility to cefoxitin, oxacillin, linezolid, clindamycin and trimethoprim sulfamethoxazole was assessed by using the Kirby-Bauer technique, and for vancomycin, an E-test was performed (Biomerieux®).
Results: 72 strains of S. aureus were isolated from 82 medical students. 72.2 % were identified as methicillin-sensitive (MSSA) and 27.8 % as methicillin-resistant (MRSA). Four MRSA strains (20 %) showed vancomycin intermediate (VISA 4-8 µg/mL) profile, 65 % of MRSA isolates was resistant to clindamycin, 40 % to linezolid and 45 % to trimethoprim sulfamethoxazole.
Conclusions: MSSA, MRSA and VISA strains are present in nostrils and hands of our medical students, with MRSA showing high resistance levels to clindamycin, TMP-SMX and linezolid, and MSSA levels up to 45 %. These findings reiterate the need to accomplish good hands hygiene in order to minimize the spread of S. aureus in community and healthcare facilities.
KEY WORDS
Staphylococcus aureus, MRSA, Microbial drug resistance.
RESUMEN
Objetivo: establecer la prevalencia e identificar el perfil de resistencia a meticilina, vancomicina y antibióticos alternativos en aislamientos de Staphylococcus aureus provenientes de estudiantes de Medicina en rotaciones hospitalarias.
Materiales y métodos: estudio observacional transversal no aleatorizado en estudiantes de medicina en entrenamiento clínico en un hospital de tercer nivel de complejidad. Las muestras fueron tomadas de hisopados nasales y de manos y cultivadas en agar sangre. A los cocos gram positivos se les realizó pruebas de catalasa, coagulasa y siembra en agar salado manitol. La susceptibilidad a cefoxitina, oxacilina, linezolida, clindamicina y trimetoprim sulfametoxazol se efectuó mediante la técnica de Kirby-Bauer y para la evaluación de la vancomicina el método de E-test (Biomerieux®).
Resultados: 72 cepas de S. aureus fueron aisladas de manos y cavidad nasal de 82 estudiantes de medicina, 72,2 % fueron identificadas como meticilino sensibles (SAMS) y 27,8 % como meticilino resistentes (SAMR). Cuatro cepas (20 %) de SAMR mostraron ser vancomicina intermedio (SAVI 4-8 mg / mL), 65 % de los SAMR aislados fueron resistentes a la clindamicina, 40 % al linezolid y 45 % al trimetoprim sulfametoxazol.
Conclusiones: en la cavidad nasal y las manos de estudiantes de medicina están presentes cepas de SAMS, SAMR y SAVI con alto nivel de resistencia para clindamicina, TMP-SMX y linezolid en los SAMR y hasta el 45 % para los SAMS. Estos resultados reiteran la necesidad de realizar una buena higiene de manos para reducir al mínimo la circulación de S. aureus en la comunidad y en los servicios de atención de la salud.
PALABRAS CLAVES
Staphylococcus aureus, SAMR, Farmacorresistencia microbiana.
INTRODUCTION
In human body has been recognized over the time a variety of microorganisms mainly bacteria, some of them are commensals and others can cause serious diseases or death. The discovery of drugs that prevent growth or bacterial division has had a deep impact on reducing the incidence of infectious diseases, mainly bacterial diseases. Nevertheless, over the past century, it has been recognized the resistance to these drugs; between 20 to 40 % of the population served in hospitals had an antibiotic-resistant bacteria strain (1-3).
Staphylococcus aureus has been recognized as one of the microorganisms with most impact even in patients with community acquired infections. S. aureus has a remarkable pathogenicity and the ability to adapt to different conditions and avoid the effect of antimicrobial treatment, thus generating huge costs and a high rate of mortality (4,5).
S. aureus has been demonstrated in health care workers; they carry it in their hands and also could be present in medical devices. S. aureus causes about 30 deaths per day associated with risk factors such as chronic diseases, immunosuppressive drug regimens or immunosuppressive diseases, where a critical factor in morbidity and mortality is the use of inappropriate antibiotics. Methicillin-resistant, vancomycin-intermediate and a few vancomycin-resistant strains have been isolated from patients treated with glycopeptides and in patients with suspected or confirmed methicillin-resistant Staphylococcus aureus (MRSA) (6-8).
Many antibiotics such as clindamycin, trimethoprim sulfamethoxazole TMP-SMX, daptomycin and linezolid for MRSA have been tested with similar therapeutic effect to vancomycin, minimal side effects and good recovery for patients (9-13).
The intravenous vancomycin is the core treatment because other drugs such as fluoroquinolones and third-generation cephalosporins are ineffective (14). However, since 1984 it has been recognized the resistance to vancomycin; such strains are designated as vancomycin-resistant Staphylococcus aureus (VRSA), trait assigned by the British Society for Antimicrobial Chemotherapy to those strains with vancomycin minimum inhibitory concentration (MIC) greater than 8 µg/mL (1,15).
According to the Clinical Laboratory Standards Institute (CLSI, formerly NCCLS), S. aureus isolates for which vancomycin MIC are 4-8 µg/mL are classified as vancomycin-intermediate (VISA), and isolates for which vancomycin MICs are greater than 8 µg/mL are classified as vancomycin-resistant. (16). VISA have been isolated from patients with persistent bacteremia, endocarditis, central nervous system infection, septic arthritis and other infections, and some multiresistant strains (tetracyclines, macrolides, aminoglycosides and teicoplanin), including a VRSA isolated from a nephrostomy tube, exhibit a MIC profile greater than 16 µg/mL (17-23).
Several studies have found some strains of community acquired methicillin-resistant S. aureus (CA-MRSA) (24, 25). In Colombia, Sosa et al. and Villalobos et al. have showed the presence of CA-MRSA by using conventional and molecular typing in children and adults (26,27). Similarly, S. aureus is an important nosocomial pathogen with resistance to oxacillin and other antibiotics (hospital-acquired-MRSA, HA-MRSA) (28).
Asymptomatic individuals will always be an important reservoir of S. aureus strains; they dwell on skin and can be easily transmitted by fomites or direct contact with others (29).
The objective of this research is to establish the prevalence and identify the profile of resistance to methicillin, vancomycin and alternative antibiotics in Staphylococcus aureus isolates from medical students in clinical training.
MATERIALS AND METHODS
A cross-sectional observational design with non-random sampling was used in 82 medical students enrolled (VII to XII semester) with at least two-week clinical training in their main clinical facility in Bogota, Colombia, without antibiotic prescription or respiratory symptoms.
After signing the informed consent previously approved by the ethics committee, samples were taken from nasal and hands swabs (2,3,30,31) and they were processed by gram stain and cultured on blood agar (25). For beta hemolytic gram-positive cocci catalase test was performed; if it was positive, then a coagulase test was performed and then it was cultured on mannitol salt agar (32).
To evaluate the methicilin pattern of S. aureus we used cefoxitin and oxacillin discs, and susceptibility of linezolid, clindamycin, trimethoprim sulfamethoxazole (TMP-SMX) as therapeutic alternative antibiotics was assessed using the Kirby-Bauer technique (9-11,14); vancomycin resistance was tested using E-test (Biomerieux®) (33). Results were reported according to the 2011 CLSI guidelines (16,34). Incubation for all tests was performed at 35 °C under 5 % CO2 during 24 hours. In some cases, serotyping was performed with commercial antiserum (Staphytect plus® oxoid) (figure 1).
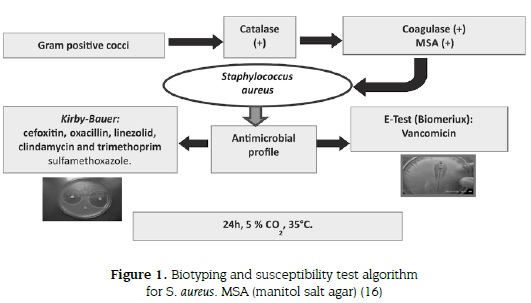
RESULTS
164 samples from students were obtained (figure 2), 72 strains of S. aureus were isolated, 47 from women (65.3 %) and 25 from men (34.7 %). 72.2 % (52 isolates) were identified as methicillin-sensitive and 27.8 % (20 isolates) as MRSA, 16 from nasal swabs and 4 from hands.
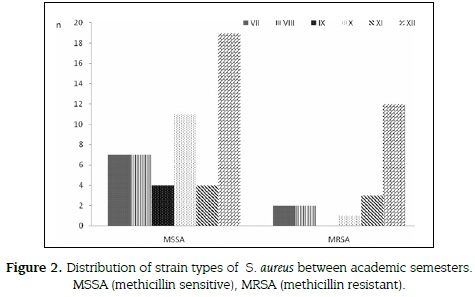
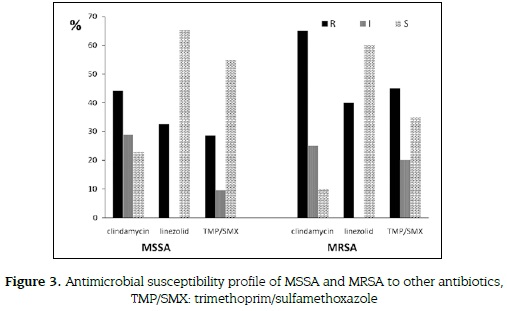
20 % of MRSA (4 of 20 strains) showed VISA (4-8 µg/mL) profile, all were isolated from nasal swabs from students up to XII semester. Out of 16 MRSA - vancomycin sensible (VSSA < 2µg/ mL) strains, 12 (60 %) were identified from nasal swabs and 4 (20 %) from hands. All Staphylococcus aureus (52 MSSA and 20 MRSA) were tested for antimicrobial susceptibility to alternative antibiotics. Thirteen (65 %) of MRSA isolates were resistant to clindamycin, 8 (40 %) to linezolid and 9 (45 %) to trimethoprim sulfamethoxazole (figure 3).
DISCUSSION
We describe the distribution of methicillin-sensitive, methicillin-resistant and vancomycin-intermediate Staphylococcus aureus strains identified in nasal swabs and hands from 82 medical students in clinical training in a tertiary healthcare facility with 350 beds and all medical and surgical facilities. Our school of Medicine in Bogota, Colombia has approximately 600 students (250 in basic sciences and 350 in clinical training).
In a previous work performed between 2009-2011, our group surveyed 155 students and obtained a total of 455 gram-positive cocci (49.7 %), 46 gram-positive bacilli (7.4 %) and 41 (6.6 %) gram-negative bacilli from hands, nostrils, oral cavity and stethoscopes. For gram positive cocci, MRSA was most prevalent with 22.7 % isolated from hands (29 %) and nostrils (23 %). Levels of 25 % resistance to ampicillin/sulbactam and 37 % to cephalexin were observed (35), Gandia et al found a 30 % of MRSA in medical students in Sinu, Colombia (36). In both cases, the rate of isolated MRSA is similar to that of this report.
Our work found four VISA strains in nasal swabs from students of last semester and it is worth noting the presence of that kind of strain in a clinical setting. On the contrary, one study in healthcare workers in Nicaragua didn't found VISA and showed a low prevalence of MRSA (<11.6 %) yet it found 15 % of multi-resistance mainly to erythromycin and clindamycin (2). In a Cuba research study it highlight the fact that medical students are continuously exposed to environment highly loaded with microorganisms, including all types of S. aureus (25).
Seventy two percent of healthcare workers from a university hospital in Bucaramanga, Colombia, were nasal carriers of S. aureus with 11.6 % of MRSA; likewise, other microorganisms such as Enterobacter aerogenes (6 %), Proteus mirabilis (2.3 %), Haemophilus influenzae, Citrobacter koseri and Providencia rettgeri (1.1 %) were isolated.
Particularly regarding S. aureus, a high level of resistance to TMP-SXT (50 %) and low resistance to clindamycin (7.7 %) is remarkable (37). In that paper, a multicenter resistance study was performed between 2001 and 2009 in intensive care units, with 11.2 % of S. aureus, and 45.6 % of MRSA. Our isolates showed similar level of resistance to TMP-SXT but an important proportion of MRSA were resistant to clindamycin as well.
Continuous screening MRSA-colonized people and the eradication of microorganisms by using an appropriated hand washing technique and nasal hygiene seem to be of the utmost importance. Hand hygiene is a key component in reducing infection; however, despite of hand washing, MRSA was recovered from healthcare workers even after using alcohol, chlorhexidine, or soap and water, which indicates that S. aureus is a very persistent bacteria (38), other study shown that a 39.3 % of hands of medical students were colonized by S. aureus, and 2,1 % by MRSA, with a poor adherence to hand washing protocol (39).
More than 20 thousand isolates of Staphylococcus aureus were collected between 2004 and 2009, 41.2 % were MRSA and 58.8 % MSSA. A total of 4 % MRSA and MSSA isolates exhibited a MIC ≥2 µg/mL to vancomycin. Linezolid, minocycline, tigecycline and vancomycin were the most active agents against S. aureus; the susceptibility to vancomycin in MRSA decreased from 100 % in 2004 to 95.77 % in 2009. Similarly, in MSSA isolates, susceptibility to vancomycin decreased from 100 % in 2004 to 91.07 % in 2009. Evidence suggests that although the number of isolates of S. aureus with reduced susceptibility to vancomycin has increased significantly from 2004 to 2009, in our work, an important level of resistance of both MRSA (≥40 % to alternative antibiotic used in case of vancomycin failure -{linezolid, TMP-SMX and clindamycin}-) and MSSA (≥ 28 %) was found (13).
In their review, Matlow et al. focus on several recommendations to prevent transmission of antimicrobial-resistant bacteria in the office and clinic, such as the use of alcohol for hands, identification of colonized or infected patients, isolation of some patients (for example: those with draining abscesses or wounds, uncontained diarrhea, fecal incontinence or cystic fibrosis), routine hand-hygiene practices for all patients, contact precautions improvement, general housekeeping and equipment cleaning, disinfecting and sterilizing to avoid transmission, consultation with experts such as public health officers or hospital infection control personnel (4).
Some studies have showed differences between MSSA or MRSA decolonization. Screening is useful in high-risk units to identify the reservoir and initiate contact precautions, this strategy being more effective than decolonization; however, screening and decolonization will be more important to prevent infection risk in MRSA carriers (40).
CONCLUSIONS
Our results indicate that MSSA, MRSA and VISA strains are present in an important proportion in nostrils and hands of our medical students. It highlights the high MRSA resistance level, between 40 %-65 % to clindamycin, TMP-SMX and linezolid, and MSSA resistance, up to 45 %. Patients and health care workers are commonly recognized as a source of pathogenic microrganisms, but medical students had not been recognized as performers in MRSA transmission.
A controversial issue is the effectiveness of routine surveillance cultures in order to detect MRSA colonized patients, healthcare workers and now, medical students, and the role of decolonizing agents for example with mupirocine in the non-outbreak clinical setting (4). These findings reiterate the need to accomplish a good personal hygiene in order to minimize the spread of pervasive, persistent and pathogenic S. aureus in community and healthcare facilities. Finally, medical students should be included in hospital infection control programs.
ACKNOWLEDGMENTS
We would like to thank the students of the School of Medicine, Universidad Militar, Bogota, Colombia. We are grateful to the technicians Iveth Hernandez and Luz Vargas for their helpful support in laboratory testing. Finally, we thank Dr. Nelida Forero Cubides for her helpful review of the English manuscript.
FUNDS
This work was supported by Grant MED 854 and 855 from Researches Fund of Universidad Militar, Bogota, Colombia.
DISCLOSURE
None of the researchers have conflict of interest. This work was presented in slide session C2-195 at 52th ICAAC meeting, San Francisco, USA, September 9-12th 2012.
REFERENCES
1. Rebiahi S, Abdelouahid D, Rahmoun M, Abdelali S, Azzaoui H. Emergence of vancomycin-resistant Staphylococcus aureus identified in the Tlemcen University Hospital (North-West Algeria). Médecine et maladies infectieuses 2011; 41:646-651. [ Links ]
2. Cáceres M. Frecuencia de portadores nasales de Staphylococcus aureus resistente a meticilina en personal de salud de hospitales de Nicaragua. Rev Panam Salud Pública 2011; 30(6):610-614. [ Links ]
3. Seybold U, Schubert S, Bognera J, Hogardt M. Staphylococcus aureus infection following nasal colonization: an approach to rapid risk stratification in a university healthcare system. J Hosp Infect 2011; 79(1):297-301. [ Links ]
4. Matlow A, Morris S. Control of antibiotic-resistant bacteria in the office and clinic. CMAJ 2009; 180(10):1021-1024. [ Links ]
5. Plata K, Rosato A, Wegrzyn G. Staphylococcus aureus as an infectious agent: overview of biochemistry and molecular genetics of its pathogenicity. Acta Biochem Polon 2009; 56(4):597-612. [ Links ]
6. Paul M, Kariv G, Goldberg E, Raskin M, Shaked H, Hazzan R, et al. Importance of appropriate empirical antibiotic therapy for methicillin- resistant Staphylococcus aureus bacteremia. J Antimicrob Chemother 2010; 65: 2658-2665. [ Links ]
7. Rehm S, Tice A. Staphylococcus aureus: Methicillin-Susceptible S. aureus to methicillin-resistant S. aureus and vancomycin-resistant S. aureus. Clin Infect Dis 2010; 51(S2):176-182. [ Links ]
8. Liu C, Chambers H. Staphylococcus aureus with heterogeneous resistance to Vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob Agents Chemother 2003; 47(10):3040-3045. [ Links ]
9. Frei C, Miller M, Lewis II J, Lawson K, Peddaiahgari R. Talbert R. Retrospective cohort study of hospitalized adults treated with vancomycin or clindamycin for methicillin-resistant Staphylococcus aureus skin infections. Clin Ther 2010; 32 (12):2024-2029. [ Links ]
10. Itani K, Dryden M, Bhattacharyya H, Kunkel M, Baruch A, Weigelt J. Efficacy and safety of linezolid versus vancomycin for the treatment of complicated skin and soft-tissue infections proven to be caused by methicillin-resistant Staphylococcus aureus. Am J Surg 2010; 199(6):804-816. [ Links ]
11. Balkhair A, Al Muharrmi BZ, Darwish L, Farhan H, Sallam M. Treatment of vancomycin-intermediate Staphylococcus aureus (VISA) endocarditis with linezolid. Int J Infect Dis 2010; 14:e227-e229. [ Links ]
12. Appleman M, Citron D. Efficacy of vancomycin and daptomycin against Staphylococcus aureus isolates collected over 29 years. Diagnostic Microbiol Infect Dis 2010; 66:441-444. [ Links ]
13. Hawser S P, Bouchillon SK, Hoban DJ, Dowzicky M, Babinchak T. Rising incidence of Staphylococcus aureus with reduced susceptibility to vancomycin and susceptibility to antibiotics: a global analysis 2004-2009. Intl J Antimicrob Agents 2011; 37:219-224. [ Links ]
14. Mamani E, Luján D, Pajuelo G. Perfil de sensibilidad y resistencia de Staphylococcus aureus. Experiencia en el Hospital Nacional Hipólito Unanue. Annal Fac Med Lima 2006; 67(2):120-124. [ Links ]
15. Palazzo I, Araujo M, Darini A. First report of Vancomycin- resistant staphylococci isolated from healthy carriers in Brazil. J Clin Microbiol 2005; 43(1):179-185. [ Links ]
16. Clinical and Laboratory Standards Institute. Section 2C Performance standards for antimicrobial susceptibility testing; twenty-first informational supplement. 2011; 31(1):68-83. [ Links ]
17. Weigel L, Donlan R, Shin D, Jensen B, Clark N, McDougal L, et al. High-Level Vancomycin-resistant Staphylococcus aureus isolates associated with a polymicrobial biofilm. Antimicrob Agents Chemother 2007; 51(1):231-238. [ Links ]
18. Horne C, Howden P, Grabsch A, Graham M, Ward B, Xie S, et al. Prospective comparison of the clinical impacts of heterogeneous vancomycin-intermediate methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-susceptible MRSA. Antimicrob Agents Chemother 2009; 53(8):3447-3452. [ Links ]
19. Howden B, Johnson P, Ward P, Stinear T, Davies J. Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2006; 50(9):3039-3047. [ Links ]
20. Hageman J, Patela J, Franklin P, Miscavish K, McDougal L, Lonswaya D, et al. Occurrence of a USA300 vancomycin-intermediate Staphylococcus aureus. Diagnostic Microbiol Infect Dis 2008; 62:440-442. [ Links ]
21. Hsueh P, Lee S, Perng C, Chang T, Lu J. Clonal dissemination of meticillin-resistant and vancomycin-intermediate Staphylococcus aureus in a Taiwanese hospital. Intl J Antimicrob Agents 2010; 36:307-312. [ Links ]
22. Tenover F, Weigel L, Appelbaum P, McDougal L, Chaitram J, McAllister S, et al. Vancomycin-Resistant Staphylococcus aureus Isolate from a patient in Pennsylvania. Antimicrob Agents Chemother 2004; 48(1):275-280. [ Links ]
23. Naesens R, Ronsyn M, Druwé P, Denis O, Leven M, Jeurissen A. Central nervous system invasion by community acquired meticillin-resistant Staphylococcus aureus. J Med Microbiol 2009; 58:1247-1251. [ Links ]
24. Teglia O, Gregorini E, Notario R, Fay F, Casellas JM, Casellas JM. Staphylococcus aureus meticilino-resistente, emergente de la comunidad. Rev Med Rosario 2007; 73:76-81. [ Links ]
25. Hernández I, Toraño G, González M, González I. Staphylococcus aureus resistente a la meticilina: detección de portadores entre niños hospitalizados y niños sanos de la comunidad. Rev Cubana Med Trop 2003; 55(3):153-61. [ Links ]
26. Sosa L, Machuca M, Sosa C, González C. Infecciones por Staphylococcus aureus meticilino resitente en niños en Bucaramanga Colombia. Salud UIS 2010; 42: 248-255. [ Links ]
27. Villalobos A, Díaz M, Barrero L, Rivera S, Henríquez D, Villegas M, Robledo C. Tendencias de los fenotipos de resistencia bacteriana en hospitales públicos y privados de alta complejidad de Colombia. Rev Panam Salud Pública 2011; 30(6):627-633. [ Links ]
28. Spirandelli K, Mamizuka E, Gontijo P. Methicillin/Oxacillin-resistant Staphylococcus aureus as a hospital and public healt threat in Brazil. Brazilian J Infec Dis 2010; 14(1):71-76. [ Links ]
29. Morell E, Balkin D. Methicillin-resistant Staphylococcus aureus: A pervasive pathogen highlights the need for new antimicrobial development. Yale J Biol Med 2010; 83: 223-233. [ Links ]
30. Rafee Y, Abdel-Haq N, Asmar B, Salimnia T, Vidaillac C, Rybak MJ, et al. Increased prevalence of methicillin-resistant Staphylococcus aureus nasal colonization in household contacts of children with community acquired disease. BMC Infect Dis 2012; 75(1):12-45. [ Links ]
31. Creamer E, Dorrian S, Dolan A, Sherlock O, Fitzgerald-Hughes D, Thomas T, et al. When are the hands of healthcare workers positive for meticillin-resistant Staphylococcus aureus? J Hosp Infect 2010; 75(1):107-111. [ Links ]
32. Palavecino E. Métodos recomendados para el estudio de susceptibilidad en Staphylococcus aureus, Staphylococcus coagulasa negativa y Staphylococcus saprophyticus: Nuevos puntos de corte e interpretación de resultados. Rev Chil Infect 2002; 19(2):119-124. [ Links ]
33. Jaramillo S. Prueba Épsilon (Etest). Rev CES Med 1998; 12(1):34-41. [ Links ]
34. Malhotra-Kumar S, Haccuria K, Michiels M, Ieven M, Poyart C, Hryniewicz W, et al. Minireview. Current trends in rapid diagnostics for methicillin-resistant Staphylococcus aureus and glycopeptide-resistant Enterococcus species. J Clin Microbiol 2008; 46(5):1577-1587. [ Links ]
35. Méndez IA, Calixto OJ, Becerra WA, Vásquez J F, Bravo JS, Pachón D P. Microrganismos presentes en fonendoscopios, manos, cavidad oral y nasal de estudiantes de una facultad de Medicina. Rev MED 2012. 20(1):90-100. [ Links ]
36. Gandia JA, Benjumea Y, Mangones LM, Villacob K P, Sánchez L, Mosquera E. Prevalencia de Staphylococcus aureus meticilino resistente en estudiantes de medicina en la Universidad del Sinú. Primer Encuentro nacional de semilleros de investigación - facultades de Medicina, 2012 julio 25-27, Bogotá, Colombia. [ Links ]
37. Espinosa C, Romero M, Rincón G, Bohórquez M, Arámbula A. Portadores nasales de Staphylococcus aureus en personal que labora en un hospital de Santander. Salud UIS 2011; 43(2):111-117. [ Links ]
38. Creamer E, Dorrian S, Dolan A, Sherlock O, Fitzgerald-Hughes D, Thomas T et al. When are the hands of healthcare workers positive for meticillin-resistant Staphylococcus aureus? J Hosp Infect. 2010; 75:107-111. [ Links ]
39. López-Aguilera S, Goñi-Yeste MD, Barrado L, González-Rodríguez-Salinas MC, Otero JR, Chaves F. Staphylococcus aureus nasal colonization in medical students: Importance in nosocomial transmission. Enferm Infecc Microbiol Clin. 2013 Jan 22. doi:pii: S0213-005X(12)00445-4. [ Links ]
40. Lucet J, Regnier B. Screening and decolonization: Does methicillin- susceptible Staphylococcus aureus hold lessons for meticillin- resistant S. aureus? Clin Infec Dis 2010; 51(5):585-590. [ Links ]













