Introduction
Infection with human immunodeficiency virus (HIV) gradually destroys the immune system, increasing the susceptibility to opportunistic infections and the development of acquired immunodeficiency syndrome (AIDS) 1. According to the 2013 reports of the World Health Organization (WHO) and the Joint UN Programme on HIV / AIDS (UNAIDS), 35.3 million people were living with HIV worldwide at the end of 2012, of which 3.3 million were children and 17.7 million were women 2,3.
In Colombia, HIV behaves as a concentrated epidemic because its prevalence in risk groups; men who have sex with men, intravenous drug users or sex workers exceeded 5%, for 2012. It was reported that the death rate in men was 7.3 and 2.2 per 100.000 women 4-7. According to Departmento Administrativo Nacional de Estatisticas (DANE) data, mortality rate from AIDS in Colombia in the same year was 4.76 per 100.000 inhabitants 4.
Proper nutrition and optimal levels of zinc are required to have a competent immune system. Zinc deficiency reduces the production of T cells and humoral and cellular immunity that can lead to increased opportunistic infections 8. The essential role of zinc and its deficiency in humans was recognized in the 1960s. Multifunctional zinc has been reported as an antioxidant in cell cultures and animal models and zinc supplements delay the progression of HIV / AIDS supported in taking antiretroviral therapy (ART) 9,10.
Oxidative stress has been recognized as a major contributing factor in many chronic diseases attributed to aging, such as atherosclerosis, cardiovascular related disorders, mutagenesis, cancer, neurodegeneration and immune disorders and even the aging process itself 10.
Currently, the World Health Organization estimates that about two billion of subjects in the developing world may be deficient in zinc. Clinical manifestations of zinc deficiency include growth retardation, testicular hypofunction, immune dysfunction, increased oxidative stress and increased generation of inflammatory cytokines 11. In the initial HIV therapy, the use of an inhibitor of non-nucleoside reverse transcriptase nucleoside (NNRTI), protease inhibitor boosted with ritonavir (IP) is recommended, along with a fixed-dose combination of nucleoside or nucleotide reverse transcriptase inhibitors (NRTIs) 12,13.
The effectiveness of NNRTI-based regimens, however, is threaten by the presence of drug resistance among new HIV infections or newly diagnosed subjects. For patients receiving antiretroviral therapy, immune failure has been defined as an inadequate increase in CD4 lymphocytes 14,15.
Antiretroviral therapy (HAART) has significantly improved the prognosis and life expectancy of people infected with the human immunodeficiency virus (HIV). The recovery of CD4 cells in treated people is accompanied by an improved CD4 response that ensure an adequate protection against opportunistic infections, however, immune recovery shows great variability from patient to patient, and the reasons for this variation are still unknown 16,17.
The long-term recovery of CD4 lymphocytes is of great clinical importance because immune deficiency is associated with twice the relative risk of clinical progression and increased mortality risk 18.
Scientific papers have linked zinc deficiency with decreased CD4 lymphocytes and decreased survival; Baum et al. showed in 2010 and in 2013 that supplementation with antioxidant, vitamins and minerals was associated with longer survival and fewer immunological failure 19,20.This study addresses the need to have local data on the role of zinc in maintaining the integrity of the immune system, disease progression to AIDS, related mortality and the need to develop an optimal zinc therapy for infected adults; then the aim of this study was to assess the benefits and safety of zinc supplements at nutritional doses in the recovery of CD4 lymphocytes.
Materials and methods
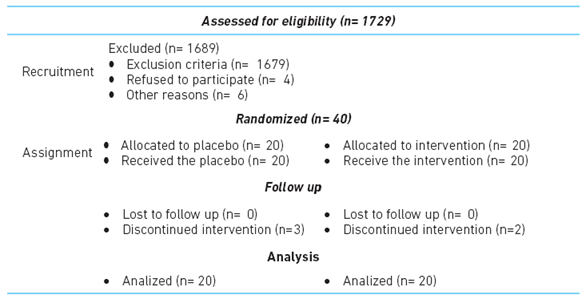
A double-blind, randomized, placebo-controlled clinical trial, involving an intervention over 12 weeks. Eligibility criteria were: (i) age ≥ 18 years, (ii) documented HIV infection (iii) receiving first and second scheme antiretroviral treatment (reverse transcriptase inhibitor nucleoside or nucleotide analogue -NA- non-nucleoside reverse transcriptase inhibitor -NNRTIs-, protease inhibitor -PI- boosted with ritonavir -PI / r-, protease inhibitors -IP-, defined according to clinical characteristics of subjects, and (iv) attending a clinical setting in Medellin, Colombia.
Subjects were excluded if uncontrolled virus (viral load greater than 40 copies per milliliter), immune recovery or did not wish to participate in the study. Achieving 40 patients for the study, belonging to one health institution of the city of Medellin - Colombia (table 1).
A sample size calculation with 80% power and 95% confidence was performed according to Baum et al. 20, where incidence of immune recovery was 76% in the group receiving zinc supplementation and 26% in the placebo group; resulting in a total sample of 38 patients, which was increased to 40 patients to be distributed between the intervention and the placebo group, in a 1:1 ratio.
Simple random assignment was made though the Epidat program. According to this randomization, laboratory bottles were marked and assigned the corresponding number, thus establishing the double-blind for researchers and patients; this blind was preserved until the intervention ended; personnel who performed the statistical analysis remained blinded throughout the analysis.
The 40 patients were assessed by a physician, master in HIV, to evaluate their medical condition; also by a pharmaceutical chemist to closely monitor adherence to treatment and to measure possible side effects.
A registered nutritionist evaluated patients in order to assess naturally possible differences in diet quality, because they all received nutritional counseling, focused on foods that could provide zinc. It should be noted that not great changes in the diet of patients were suggested, but whether they were taking other nutritional supplements (defined as an exclusion criterion for this research) and any food that could inhibit the absorption as high phytate consumption was monitored.
During the 12 weeks follow-up, assigned treatments were provided to 40 subjects: group 1: 20 patients receiving placeb, and group 2 with 20 patients receiving zinc sulfate (sulzinc®).
Both compounds were introduced into syrup; they were similar in color, odor, flavor (grape) and packaging. Humax Pharmaceutical® was responsible for the development in one lot. A daily dose of 20 mg of zinc sulfate to patients allocated to treatment group was provided.
It was considered significant an increase in CD4 lymphocytes greater or equal to 20% between the groups. recruitment between October 2013 and April 2014.
In the medical and nutritional consultations social, demographic and clinical variables were registered . Errors and biases established on non-nutritional intake at the time of entrance to the study were monitored; the information bias was attempted to control through getting of the information from primary source. Likewise, the selection bias was controlled including patients who met the inclusion criteria and finally, confounding was controlled matching with controls by potential confounding variables as well by adjusted analysis with it ones.
An intent-to-treat analysis was performed. Patients who were lost to follow-up were included and the same initial CD4 count was considered the worst-case scenario, as it was intended to assess the effectiveness of the intervention.
Continuous variables were summarized using the corresponding means with standard deviation and categorical variables with frequencies and proportions. To determine statistical differences between the outcome and independent variables, t- test or U test of Mann-Whitney were used. Association of categorical variables was assessed using chi-square or Fisher's exact test, when appropriated.
Pre and post laboratory markers measurements were compared using Wilcoxon test. In all cases a two-sided P value of 0.05 was considered statistically significant. Relative risks were calculated with 95% confidence intervals. All statistical analyses were performed using IBM SPSS Statistics ® (version 22.0, Armonk, NY: IBM Corp.) This study was approved by the Institutional Ethics Committee of the Universidad CES (record 53, 160 of 10/18/2012) in Medellin, Colombia and all subjects gave informed consent. The ethical standards of the Declaration of Helsinki and the considerations of Article 11 of Resolution 8430/1993 by the Ministry of Health of Colombia were followed, ethical principles were considered autonomy, beneficence, non-maleficence and justice.
Results
The mean patient age was 49.4 ± 9.5 years, no statistical differences were found between groups regarding age (p = 0.62), 85% of the research subjects were males and no statistical differences were found between groups by sex. When other sociodemographic variables were compared, no statistical differences were found (Table 2).
Table 2 Subject characteristics
| Placebo n= 20 | Intervention group n= 20 | P value | |
|---|---|---|---|
| % | % | ||
| Sex (male) | 85 | 85 | 1 |
| Socioeconomic status* | |||
| 1 | 15 | 10 | 0.64 |
| 2 | 50 | 50 | 1 |
| 3 | 35 | 30 | 0.74 |
| 5 | 0 | 10 | ---- |
| Marital status* | |||
| Single | 75 | 85 | 0.43 |
| Married/cohabitation | 25 | 15 | 0.43 |
| Educational level* | |||
| Elementary/uncomplete elementary | 35 | 45 | 0.51 |
| Hihg school/uncomplete high school | 60 | 30 | 0.06 |
| Technician/Undergraduate school | 5 | 25 | 0.08 |
| Social security scheme* | |||
| Subsidized | 65 | 60 | 0.74 |
| Contributory | 35 | 40 | 0.74 |
*chi-square, statistically significant p<0.05
Intervention group had a higher average weight (72.4 ± 11.6) compared to the control group, with statistically differences (p = 0.01), which was confirmed by nutritional indicators assessment at baseline, finding in the intervention group a highest percentage of overweight (table 3).
Table 3 Nutritional characteristics
| Placebo | Intervention | Total | P value | |
|---|---|---|---|---|
| n=20 | n=20 | n=40 | ||
| Age * | 48.7 ± 9.4 | 50.2 ± 9.7 | 49.45 ± 9.5 | 0.62 |
| Weight* | 63.3 ± 11.4 | 72.4 ± 11.6 | 67.82 ± 12.3 | 0.01 ** |
| Height* | 1.66 ± 0.06 | 1.67 ± 0.08 | 1.66 ± 0.07 | 0.58 |
| BMI+* | 22.9 ± 3.1 | 25.8 ± 3.5 | 24.3 ± 3.6 | 0.00 ** |
+ BMI: body mass index. *Ẋ: mean; SD standard deviation; T student.
** Statistically significant p <0,05
In the beginning, it was found that 50% patients had a CD4 cell count of 159 or less (interquartile range -IQR- = 73.75) with a maximum value of 853 and a minimum of 51. Also the final CD4 count was evaluated and it was found that 50 % of patients had 195 or less (IQR = 158) with a maximum value of 1042 and a minimum of 73 (Mann Whitney U test p = 0.85). By comparing the variation of CD4 lymphocytes at baseline and post intervention in each group, it was found that there were variations in the number of CD4 cells (p = 0.00). After the intervention, an increase ≥ 20% in CD4 lymphocytes was compared between groups, and a better response was observed in patients who received zinc (90% vs. 25%, p = 0.00). (Table 4)
Table 4 Clinical characteristics according to study group
| Placebo | Intervention | P value | |
|---|---|---|---|
| Years from diagnosis* | |||
| 4 (10) | 3.50 (2.75) | 0.27 | |
| Baseline CD4 * | |||
| 163 (56) | 157 (86.75) | 0.85 | |
| Post intervention CD4 * | |||
| 145.50 (91) | 253 (108.5) | 0.00‡‡ | |
| Change CD4** | |||
| 7,04 ± 28.22 | + 61.17 ± 37.79 | 0.00‡‡ | |
* Me (IQR); U Mann-Whitney p <0,05 ‡‡.
** Ẋ: mean; SD standard deviation; T student p <0,05 ‡‡.
In the group receiving zinc sulfate, 18 subjects showed variation above than 20% in CD4 lymphocytes level, and just 5 in the control group, RR = 3.6 (95% CI 1.66 - 7.8; p = 0.000) and reduction of relative risk of 74% compared to placebo. It is needed to treat two HIV patients to have an increase in CD4 (NNT ≈ 2).
Three patients in the intervention group (15 %) presented some type of adverse event during the observation period: abdominal pain, headache, nausea and diarrhea. No adverse effects were seen in the control group.
In the assessment for immune recovery, significant differences were not found between treatments administered to the groups (p> 0.05)
Discussion
This study presents a comparative experiment between the zinc sulfate supplement (Sulzinc®) and placebo in 40 adult patients with HIV / AIDS, who had immune failure at the time of the study. The results of this study show an increase in CD4 count for zinc supplemented group compared to the placebo group after 12 weeks. This effect on the variation of CD4 is not only statistical, but also clinically and socially significant. Our results are similar to Rodriguez´s, where she describes that zinc suplementation in a daily basis could reduce the susceptibility to infections in HIV immunocompromised patients, preventing the virus replication, and then the damage in celular immunity or making the apoptotic process 21.
From a clinical point of view, an immune recovery is reflected in a better response to opportunistic infections, could favor decreasing the risk of disease. Socially, it has been found that the occurrence of opportunistic diseases cause economic losses in terms of development and productivity, generating physically challenged adults with low levels of productivity 22.
Daily supplementation with zinc sulfate has been shown to help improve the recovery of CD4 in patients with HIV/AIDS, reducing the incidence of opportunistic infections, analogous to the results of this study, since patients receiving sulfate zinc showed a decreased risk.
Asdamongkol et al. 23 conducted a study with 31 patients who were considered immune - discordant (defined as CD4 counts below 200 cells / L or lower annual increase of 30% from baseline after receiving 12 months of antiretroviral therapy), finding that the CD4 count increases significantly after administration of 15 mg a zinc supplement. In accordance with the present study in which a significant increase in final CD4, after supplementation of zinc sulphate with a reference value of 20%, established by clinical criteria based on the duration of the intervention, as other authors suggested 13-15.
In the present study the delivered dose of zinc sulfate was 20 mg daily for 12 weeks, according to the recommendation adopted by Instituto Nacional de Vigilancia de Alimentos y Medicamentos (INVIMA) in Colombia, for the management of diarrhea and the daily doses of zinc supplements suggested by the International Zinc Nutrition Consultative Group (IZiNCG, for its acronym in English).
The Working Group on Treatment of HIV / AIDS (Spain) 24 refers to the use of zinc supplements to stimulate the rise of CD4 levels in people with HIV / AIDS; it has been discussed about taking zinc supplements and the effect it may have on slowing the progression of HIV / AIDS, defining zinc as a micronutrient and associating its deficiency with immunosuppression favoring the progression of opportunistic diseases.
It has been shown that immune recovery of patients diagnosed with HIV/AIDS, may be adversely affected by advanced age, male sex and type of ART used 25,26, in opposition to the findings in this study, where it was not found a relation between age, sex or type of therapy.
It has been demonstrated that the increase in CD4 can be influenced by the type of medicament therapy (analog or non-nucleoside reverse transcriptase inhibitor and protease inhibitors 27, as opposed to the study of Campa et al. in which no significant differences with the type and duration of antiretroviral therapy were found. In this study, findings did not show a statistically significant difference28.
The main finding of this study is consistent with some research which reported a greater effect on the variation of CD4 in the intervention group with zinc sulfate compared with the placebo group. Our results coincide with some studies using different interventions 29, supporting the importance of an appropriated zinc consumption because it affects specific and non-specifc immune function. (30).
Zinc is a trace mineral that has been recognized as an important in maintaining a healthy immune system factor. Low levels of zinc in plasma predict a threefold increase in mortality related to HIV, while adequate levels of the mineral in the blood have been associated with a slow disease progression and a decrease in the rate of infections opportunistic 31.
An adequate zinc level is critical for immune function. Zinc acts as an antioxidant and may protect cells from oxygen radicals produced during the nonspecific immune response 32.
Limitations
Our results cannot be generalized to the entire population of infected patients in Medellin, they are only applicable to the population where the study was performed. Another limitation of the study was that a measurement of blood levels of zinc was not carried out to determine if there was deficiency.