Introduction
The Zika virus is a Flavivirus that recently caused serious epidemics in Brazil, Colombia and other countries of the continent. Between August 2015 and December 2016, 106.000 Zika cases were notified in Colombia 1. After Brazil, Colombia has been the most-affected country, with the highest number of probable cases reported du ring Zika virus outbreak in South America 2. Although it has been widely reported that approximately 80% of people with the virus infection are asymptomatic, Zika virus infection during pregnancy is a cause of microcephaly and other congenital abnormalities in the developing fetus and newborn and it may also be a trigger for Guillain-Barré syndrome in adults 3,4. In Colombia, 48% of the infants and fetuses with microcephaly reported after epidemic were positive for Zika virus infection 5.
This virus is transmitted to humans mainly by the bite of infected Aedes mosquitoes, but there is also evidence of infection by sexual transmission and congenital cases 6,7.
The first detection of the Zika virus in mosquitoes was in Aedes africanus8. Then, the epidemic in the rural area of the Yap Islands in 2007 was attributed to Ae. hensilli9 and Ae. aegypti was the main vector in the French Polynesia outbreak of 2013 10.
Although Ae. aegypti is considered the most important vector for the Zika virus trans mission to humans, Ae. albopictus was associated as vector in the Gabon outbreak (Central Africa) in 2007 11 and recent studies have found the virus in Culex mos quitoes 12,13.
Despite the natural infection of mosquitoes with Zika virus has been reported, few studies have been carried out 14,15,16,17. The purpose of this study was to surveillance the presence of Zika virus in field-collected mosquitoes Ae. aegypti, Ae. albopictus, and Culex spp during a year after the epidemic in Colombia.
Methods
The study area was the city of Medellín (Antioquia - Colombia) which is the second largest in the country. Mosquitoes were captured with entomological aspirators on a monthly basis between March and September of 2017, in four houses in around each of the 250 entomological surveillance traps installed by the Secretaria de Salud de Medellin. Additionally, 70 Educational Institutions and 30 Health Centers were visited each month. Live mosquitoes were transported to the Medical Entomology Laboratory of the Faculty of Medicine of Universidad de Antioquia for taxonomic identification. Pools were formed containing one to 10 mosquitoes, which were analyzed by reverse transcription polymerase chain reaction (RT-PCR) for the detection of Zika virus.
Ribonucleic acid (RNA) extraction was performed using the commercial kit RNeasy Mini Kit® (Qiagen). Each pool was mechanically macerated following the protocol recommended by Qiagen. One-step RT-PCR was performed with the Luna Universal One-Step RT-qPCR kit ® (Biolabs). Each 10μl reaction contained 1 μL of RNA, 1X enzyme mix, 1X reaction mix and 0.4 μM of each of the primers ZIKF-5’-CCTTGGATTCTTGAACGAGGA-3’ and ZI KR-5’-AGAGCTTCATTCTCCAGATCAA-3’ 18. These primers amplified a specific region of the NS5 gene of the virus of approximately 191 bp.
For the RT-PCR the following thermal profile was used: reverse transcription at 55°C for 10 min, initial denaturation at 95°C for 1 min, followed by 40 cycles at 95°C for 10s, 56°C for 30s and 72°C for 20s. Negative controls for extraction and amplifica tion were included in each reaction, and virus RNA from the supernatant of infected cells was included as a positive control. The controls were processed simultaneously and under the same conditions as the samples. The amplification products were analyzed on 2.5% agarose gels in 0.5X Tris Borate EDTA (TBE), stained with Ethidium Bromide (0.6μg/ml) and visualized under (ultraviolet) UV light.
To confirm the results of the pools, positive samples were randomly selected and se quenced in Macrogen (Seoul, Korea). Forward and reverse sequences were manually edited and aligned using the BioEdit v.7.2.5 program. The sequences obtained were compared with a reference sequence (KY785466.1) from GenBank, which belongs to a Colombian isolate of the Zika virus, and were subsequently compared with the Na tional Center for Biotechnology Information (NCBI) database using the Blastn algori thm to estimate the identity percentages with reported sequences of the Zika virus. This was a descriptive study conducted with full compliance with ethical standards of research. We explained the experimental procedure to the community and ob tained consent from residents to collect mosquitoes. This study was classified as risk-free research according to resolution number 8340 (1993) from the Ministerio de Salud y Protección Social of Colombia.
Results
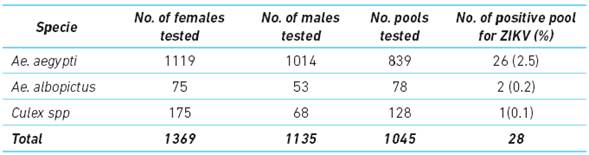
A total of 2504 mosquitoes were analyzed (Ae. aegypti, Ae. albopictus and Culex spp). A subset of mosquitoes was dissected to separate the abdomen section and the secondary tissues such as the head and thorax to identify the disseminated form of the virus. From the 1 045 pools evaluated, 2.8 % were found to be positive for Zika virus (Table 1).
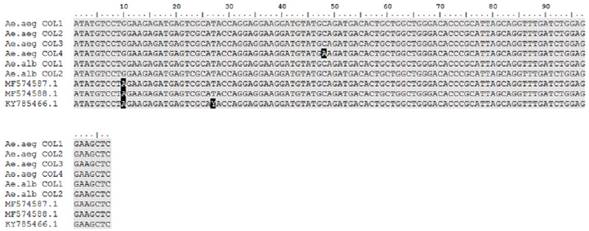
The sequences obtained presented identity values greater than 96%. The nucleotide sequence of the Cx. quinquefasciatus pool showed high identity percentage with the isolates from Haiti (MF384325.1), Brazil (MF376166.1) and Colombia (MF574587.1) (Figure 1).

The MF384325.1 sequence correspond to isolate from Haiti, MF376166.1 sequence correspond to isolate from Brazil; and MF574587.1 sequence to isolate from Colombia.
Figure 1 Nucleotide sequence alignment of Zika virus found in Cx. quinquefasciatus
On the other hand, the nucleotide sequences of Ae. albopictus and Ae. aegypti pre sented identity percentages around 98% with isolates from Colombia (MF574587.1, MF574588.1 and KY785466.1) (Figure 2).
Discussion
This is the first report of natural infection of Zika virus in Aedes and Culex mos quitoes in Colombia. Similar results for Aedes mosquitoes have been registered in Malaysia 14 and recently in Senegal 15, Brazil 17 and Mexico 19. For the case of Cx. quinquefasciatus, there is controversy with the incrimination of this mosquito as Zika vector. Some laboratory research has shown that this species is not com petent to transmit the virus 17,20,21,22, but recent studies have concluded that Cx. quinquefasciatus could have a role in the transmission. In Brazil, the presence of the virus was detected in mosquitoes captured in the areas of incidence of the di sease and in laboratory conditions, artificially-fed Cx. quinquefasciatus mosquitoes were able to replicate the virus in the midgut, salivary glands and saliva 13. These results are similar to those previously reported in China 12.
It is important to consider that, according to the feeding habits of Cx. quinquefasciatus, it is possible that our results are due to recent feeds on viraemic hosts. Therefore, future studies are necessary to determine the vectorial competence of this species in the transmission of the Zika virus.
Regarding the infection with Zika virus in Ae. albopictus, it has been previously demons trated that this species is a competent vector for the virus under laboratory condi tions 23,24, and is considered the main vector in some places like Gabon 11 and Sinagapur 25.
Our results do not allow the incrimination as vector of Ae. albopictus in the transmission of zika. However, finding it naturally infected in head and thorax, highly suggests that it plays a role in the transmission of this arbovirosis. Therefore, the monitoring of this species should be considered in anti-vector programs and subsequent studies should be conducted to determine its vector role in the transmission of Zika and new arboviruses.
Conclusion
The results obtained in this study evidence the importance of the virological survei llance of mosquitoes and the need to incorporate it into public control programs. This also implies a redesign of the surveillance and control strategies for arbovirosis, due to the differences in the bionomies of these three mosquito species. These findings show the need to monitor of virus in mosquitoes in other areas of the country, which allow to determine the vectorial role of these species in Colombia.