Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Acta Neurológica Colombiana
versão impressa ISSN 0120-8748
Acta Neurol Colomb. vol.29 no.1 Bogotá jan./mar. 2013
Recibido: 27/09/12. Revisado: 11/12/12. Aceptado: 11/12/12.
SUMMARY
Introduction: cervical dystonia (CD) is a frequent disabling condition. Botulinum Toxin (BT) type A is effective for CD. BT type B is an alternative, but its use for patients who develop neutralizing antibodies against BT type A is debatable.
Objetive: the aim of this study was to compare the efficacy of BT type B (>5000 U) versus type A and placebo patients with CD.
Materials and methods: we identified RCTs comparing interventions. Quality assessment was made according to Cochrane Collaboration. A pooled analysis was conducted for continuous and dichotomous data using a random effect model identifying OR [CI95%, p < 0.05]. Primary outcomes were improvement in TWSTRS until week sixteen. Secondary outcomes were adverse effects and costs.
Results: five RCTs fulfilled inclusion criteria (217 patients treated with BT type B; 224 patients in placebo group or type A). Two trials compared BT type B versus type A and three versus placebo. Quality assessment showed high quality of trials. BT type B improved TWSTRS scores at 4 weeks versus placebo [OR: 6.54 (CI 95%: 2.68 - 10.39; p<0.00001)]. No significant difference in efficacy was found in BT type B versus type A patients [OR:-2.16 (CI 95%:-2.64-1.88; p<0.0001)]. At week sixteen, BT type B demonstrated a sustained efficacy versus placebo. Dry mouth was significantly more frequent with BT type B compared to BT type A. Adverse events such as dry mouth and dysphagia were significantly more frequent in the BT type B group when compared to placebo. There was no information about costs.
Conclusions: no significant differences in safety and efficacy were found between BT type B when compared to type A in CD patients. BT type B is more frequently associated with dry mouth than the A serotype. More studies are needed to evaluate therapeutic options in type A resistant patients. Further evidence is required to evaluate the efficacy of BT types A and B prospectively.
KEY WORDS: Cervical Dystonia, Adults, Botulinum Toxin Type B, Botulinum Toxin Type A, Botulinum Toxin Type A Resistance, Placebo, Randomized Controlled Trials, Safety, Efficacy (DeCS). (Diana M. Prada GT. Botulinum Toxin Type B vs Type A in Cervical Dystonia: A Meta - analysis of high quality trials. Acta Neurol Colomb 2013;29:27-35).
RESUMEN
Introducción: la distonia cervical (DC) es una enfermedad limitante y frecuente. La toxina botulínica (TB) tipo A es eficaz en distonia cervical. La TB tipo B es una alternativa, pero su uso en pacientes que desarrollan anticuerpos neutralizantes contra la TB tipo A es controversial.
Objetivo: comparar la eficacia de la toxina botulínica tipo B (dosis > 5000 U) versus la toxina botulínica tipo A y el placebo en pacientes con distonía cervical.
Materiales y métodos: se identificaron ensayos aleatorios controlados (ECA) que compararon las intervenciones. La evaluación de calidad fue realizada de acuerdo a la Colaboración Cochrane. Se realizó un análisis combinado para datos continuos y dicotómicos utilizando un modelo de efectos aleatorios identificando el riesgo relativo u "odds ratio" [IC95%, p<0.05]. La medida de resultado primario fue la mejoría en la escala TWSTRS hasta la semana 16. Las medidas de resultado secundarias fueron eventos adversos y costos.
Resultados: cinco ECAs cumplieron los criterios de inclusión (217 pacientes tratados con TB tipo B; 224 pacientes en el grupo placebo o TB tipo A). Dos ensayos compararon la toxina botulínica tipo B versus tipo A y tres toxina botulínica tipo B versus placebo. La evaluación de calidad mostró alta calidad de los ensayos. La TB tipo B mejoró la puntuación en la escala TWSTRS a la semana 4 versus placebo [OR: 6.54 (CI 95%: 2.68 - 10.39; p<0.00001)]. No se encontraron diferencias significativas en eficacia en pacientes tratados con TB tipo B versus tipo A [OR: -2.16 (CI 95%:-2.64-1.88; p<0.0001)]. A la semana 16, la TB tipo B demostró eficacia sostenida frente a placebo. La xerostomia fue significativamente más frecuente con TB tipo B comparada con la tipo A. Eventos adversos como xerostomia y disfagia fueron significativamente más frecuentes en el grupo de TB tipo B comparado con placebo. No se encontró información sobre costos.
Conclusiones: no se encontraron diferencias significativas en eficacia y seguridad entre la TB tipo B comparada con la tipo A en pacientes con DC. La TB tipo B se asocia más frecuentemente a xerostomia que el serotipo A. Se necesitan más estudios para evaluar opciones terapéuticas en pacientes resistentes a la TB tipo A. Se requiere mayor evidencia para evaluar de forma prospectiva la eficacia de las TBs tipos A y B.
PALABRAS CLAVE: Distonía Cervical, Adultos, Toxina Botulínica Tipo B, Toxina Botulínica Tipo A, Resistencia a Toxina Botulínica Tipo A, Placebo; Ensayos Aleatorios Controlados; Seguridad; Eficacia (MeSH). (Diana M. Prada G. Toxina Botulínica Tipo B vs Tipo A en la distonía cervical: Un meta - análisis de ensayos de alta calidad. Acta Neurol Colomb 2013;29:27-35).
INTRODUCTION
Cervical dystonia (CD) manifested as involuntary contractions of neck and shoulder muscles, is a worldwide problem and the most common form of focal dystonia in adults. It has an estimated prevalence of 11,5 cases per 100,000 individuals (1, 2) and 57 to 90 cases per million in USA and Europe (3). Chronic pain, abnormal neck posture, diminished neck mobility, and tremor are frequently encountered in patients with CD (4). CD has a negative impact in people's quality of life (QoL), with additional symptoms such as depression, pain, social embarrassment and interference in daily life activities, unless treatment is initiated (5 - 7). Benzodiazepines, muscle relaxants, anticonvulsants and anticholinergics have been used in CD (8). Anticholinergics were a first line treatment before the introduction of botulinum toxins (9, 10). Since the eighties, botulinum toxin (BT) type A has been the gold standard for treatment in CD with level A recommendation. The efficacy of BT type A has been related to significant improvement of QoL, pain reduction and a low adverse event profile, as proved in several trials including a meta-analysis (11).
It is estimated that a mean of 6,5% of patients submitted to BT type A treatment produce blocking antibodies with clinical resistance or less response after subsequent injections (12). Other series have found a rate of antibody conversion of 4,3 to 10% in CD patients injected with on a Botulinum toxin A (3). Naumann et al calculated a 1,28% (4/132) conversion to an antibody- positive status after a mean of 3,8 treatments with on a Botulinum toxin A in patients with CD (13). In patients with secondary resistance, the use of another serotype such as BT type B is indicated. The safety and efficacy of BT type B versus placebo in CD has been demonstrated in a previous meta-analysis and a small subgroup analysis showed greater efficacy in BT type A resistant versus type A non-resistant patients (14). A prospective open label trial concluded that BT type A resistant and non-resistant patients with CD improve significantly when switched to BT type B (15).
A meta-analysis from year 2003, designed to compare the efficacy between type A and B toxins in CD, made no conclusions, for the two ongoing trials identified at that time had no preliminary results to make comparisons (16). This study aimed to compare the efficacy of BT type B (>5000 U) versus type A and placebo patients with CD. Primary outcomes were the improvement in Toronto Western Spasmodic Torticollis Rating Scale scores (TWSTRS) until week sixteen. Secondary outcomes were adverse effects and costs.
MATERIALS AND METHODS
A search of randomized controlled trials comparing the use of BT type B against BT type A or placebo, published in English language since 1990 to August 2012, was conducted in databases including Pubmed, Cochrane library, Ovidsci/expanded, LILACS, EMBASE, MEDLINE, and COCHRANE. References of articles and Boolean operators were reviewed to identify other studies. Also, a review of abstracts published in scientific associations such as the American Academy of Neurology and the Movement Disorders Society, was performed. The MESH terms used in this search were "Botulinum toxin type A", "Botulinum toxin type B", "Placebo", "Treatment", "Botulinum toxin type A resistant patients", "Botulinum toxin type A non- resistant patients", "neutralizing antibodies", "cross-immune reactions", "frontalis muscle test", "Cervical dystonia", "Efficacy", "Adverse events", "Costs", "Adults", "Toronto Western Spasmodic Torticollis Rating Scale", "TWSTRS".
Inclusion criteria of trials were: all randomized-controlled trials (RCTs) comparing the efficacy of BT type B (more than 5,000 U) versus type A (resistant and non-resistant patients) or placebo in CD regardless of the number of patients or database; adult patients treated with BT for CD; over 18 years old; diagnosed with CD in the last 6 months to 1 year; exposed or not to BT type A or B treatments for CD; evaluations performed with the TWSTRS at 4 weeks for efficacy as a primary end point, and follow - up at 8, 12 and 16 weeks for sustained efficacy and safety profile; single injection of BT types A and B. Exclusion criteria were: BT type B doses minor of 2,500 U; primary resistance to BT type A; persistent neurological or neuromuscular diseases; pure anterocollis or retrocollis; serious concomitant illnesses, such as hepatic, cardiovascular, hematological, dermatological, pulmonary, psychiatric and neurological disorders; pregnancy; surgical procedures for CD, such as selective denervation or myectomy; use of drugs with interference in safety and efficacy outcome measures (benzodiazepines, muscle relaxants, narcotics).
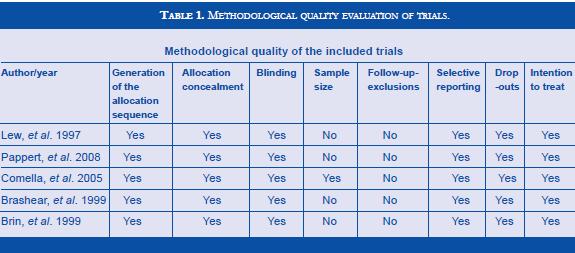
The methodological quality of the studies was assessed according to the Cochrane Manual for Systematic Review of Interventions. The following characteristics were determined in each study: generation of the allocation sequence, allocation concealment, blinding, sample size, follow-up, exclusions, intention-to-treat analysis, selective reporting, and drop-outs. All trials reviewed had high quality standards (Table 1). Data specific to the trials and characteristics of the population studied was obtained. An analysis of the trials was performed and information regarding to authors, country where it was conducted, publication year, settings, multicenter trial, participants, interventions, controls, and main outcomes, were extracted. Information about the population studied included number of patients previously exposed to BT, BT type A resistant or non-resistant at the beginning of the trial, number of patients randomized, number of patients allocated to BT type A, BT type B or placebo, number of patients not randomized, and number of patients excluded after randomization and reasons for exclusion. Results were expressed as a reduction in the TWSTRS total score. The TWSTRS is a scale designed to determine the impact of CD in patients, frequently used in CD trials, and composed of three subscales: severity, disability and pain, with 0 as the lowest or best score and 87 the highest or worse score.
Primary outcomes were efficacy of BT type B versus BT Type A or placebo patients with CD, consistent with a reduction in TWSTRS total score composed by its three subscales, at week 4 after a single injection. Secondary outcomes were reductions in TWSTRS total scores at weeks 8, 12 and 16; frequent adverse events encountered with BT type B versus placebo, and BT type B versus type A CD patients; and costs.
RCTs comparing the interventions were identified. Quality assessment was made according to the Cochrane Collaboration. A pooled analysis was conducted for continuous and dichotomous data using a random effect model identifying OR [CI 95%, p<0.05]. A statistical methods analysis was performed.
RESULTS
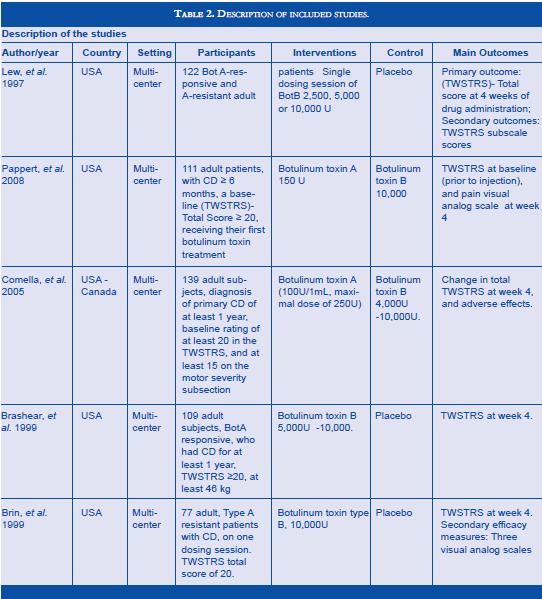
Literature search found 5 RTCs (8,12,17,18,19) which fulfilled inclusion criteria (217 patients treated with BT type B; 224 patients in placebo group or type A). Methodological quality evaluation of each trial is shown in table , and description of included trials in table 2. The studies were homogeneous in methodological criteria. Two trials compared BT type B versus type A treated patients, and there compared BT type B treated patients versus placebo. Pappert et al included 46/111 toxin naive patients with CD in the type B group (10,000 U) and 47/111 in the type A group (150 U) (17). Comella et al enrolled BT type A responders, 74/139 in the BT type A group (mean dose of 205 U) and 65/139 in the BT type B group (mean dose of 8,520 U) (18). Lew et al enrolled both BT type A resistant and non - resistant previously treated patients, 30/120 in the BT type B group and 30/120 in the placebo group. Brin et al enrolled only BT type A resistant previously treated patients, 39/77 in the BT type B group (10,000 U) and 38/77 in the placebo group (10). Brashear et al enrolled only BT type A non-resistant previously treated patients, 73/109 in the BT type B group (5,000 and 10,000 U) and 36/109 in the placebo group (19). Patients treated with doses inferior to 5,000 U were excluded from de analysis (8).
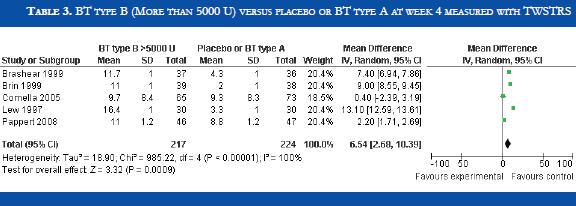
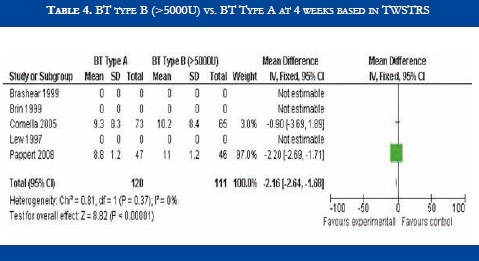
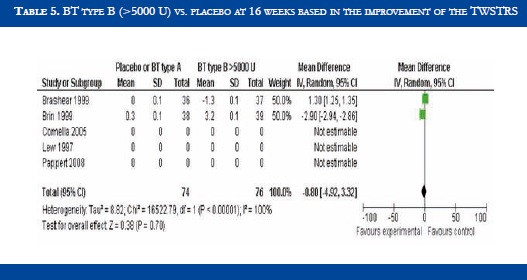
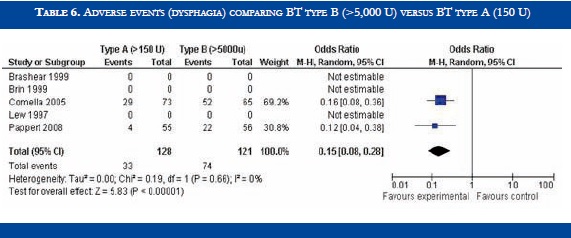
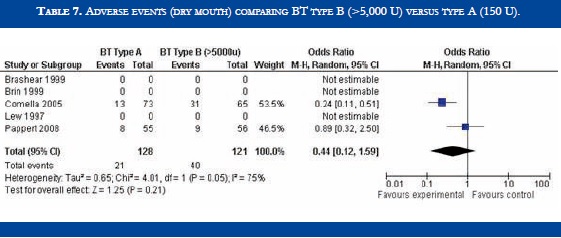
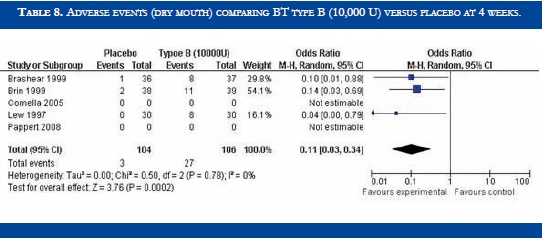
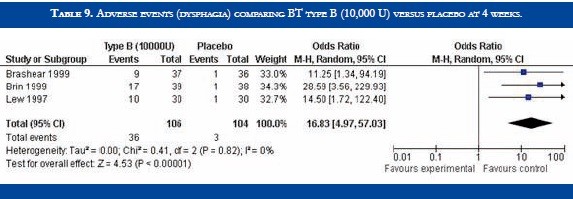
BT type B improved TWSTRS scores at 4 weeks versus placebo [OR: 6.54 (CI95%:2.68-10.39; p<0.00001)] (Table 3). No significant difference in efficacy was found in BT type B versus type A patients [OR:-2.16 (CI95%:-2.64-1.88; p<0.0001)] (Table 4). At week sixteen, BT type B demonstrated sustained efficacy versus placebo (Table 5). The risk of dysphagia was higher for BT type B compared to type A in one trial (48% against 19% respectively). In the Pappert et al trial, dysphagia was present in 1,8% of the BT type B treated patients, compared to 0% in the BT type A patients (Table 6). Dry mouth was significantly more frequent with BT type B compared to BT type A (Table 7). Adverse events such as dry mouth and dysphagia were significantly more frequent in the BT type B group when compared to placebo (Tables 8 and 9). There was no information about costs.
DISCUSSION
Cervical dystonia is a disabling condition with quality of life impairment in economically active people. Almost 70% of patients develop pain and the burden of the disease is high in developed and under developed countries. Botulinum toxin is synthesized by Clostridium botulinum an anaerobic bacterium found in the environment. There are different toxin serotypes, being the A and B the most powerful. BT type A injected in cervical muscles is the gold standard treatment for CD with a high rate of efficacy and a safe adverse event profile. The majority of patients respond satisfactorily to BT type A therapy, nevertheless some patients develop neutralizing antibodies and resistance to BT type A. In a few cases efficacy diminishes gradually with repeated injections and it is important to consider other botulinum toxin serotypes, such as the B type, when resistance is encountered. The percentage of patients resistant to BT type A treatment in cervical dystonia is estimated to be up to 6.5%. BT type B efficacy in BT type A patients with CD is an intervention where meta analysis could help detect a favorable effect especially when small and few trials have addressed this issue.
The studies found in the literature that fulfilled the inclusion criteria for this study were homogeneous, clinically and methodologically. To reduce bias in the BT type B treated group, only patients injected with more than 5,000 U of BT type B, were selected. BT type A treated patients received 100 to 250 U in each injection. Almost all patients had been previously treated with BT type A, some still responsive and others resistant, while one study selected naïve patients, and compared efficacy between BT type A versus type B. In this particular study by Pappert and cols, no significant differences in efficacy between toxins A and B, or important differences in adverse events were encountered, perhaps a slightly higher frequency of dysphagia, and more frequently dry mouth, which is consistent in other trials, and the fact of the patients being naïve did not affect the results. All patients were evaluated with the TWSTRS, and all studies had a single injection of the toxins and same follow up visits, with an efficacy evaluation at week 4, and follow up at week 16, were important issues to reduce bias.
Results from this meta-analysis demonstrate efficacy of BT type B versus placebo and similar efficacy between BT types A and B in CD patients. No significant differences in efficacy were found between BT type B when compared to type A with both serotypes obtaining favorable results. Adverse events, such as dry mouth and dysphagia, were more frequently encountered in the BT type B group when compared to placebo. BT type B is more frequently associated with dry mouth than the A serotype, and dysphagia is also slightly more frequent. Both toxins, A and B were safe and efficacious in CD patients. BT type B is preferred in resistant patients, when type A is no longer an option. Hospitals and neurologists must assess the percentage of patients resistant to BT type A to effectively design other therapeutic strategies. No information was found about costs, this being an issue of important dimensions to address in middle and low - income countries, especially when economically active populations are affected with CD. Cost effectiveness studies with different toxins are also needed in order to make decisions about high cost interventions in our health system. No information is available to make cost effective decisions.
The discussion of the best therapeutic option in type A resistant CD patients is still open. A large sample is required to perform a randomized controlled trial (RCT) with enough power to detect differences in efficacy between BT type A resistant and non-resistant patients treated with BT type B and or consider other therapeutic options. The sample sizes in the studies are small and the studies only assessed a single injection response. More studies are needed to evaluate the efficacy of BT types A and B prospectively. Larger samples of patients with CD need to be included in trials to make recommendations.
REFERENCES
1. JANKOVIC J. Treatment of dystonia. Lancet Neurology 2006; 5: 864-872. [ Links ]
2. QUEIROZ MB, CHIEN HF, BARBOSA ER. Quality of life in individuals with cervical dystonia before botulinum toxin injection in a Brazilian terciary care hospital. Arqu. Neuro - psiquiatr 2011; 6: 900-904. [ Links ]
3. ANYANWU B, HANNA PA, JANKOVIC J. Clinical Uses of Botulinum Toxins, New York: Cambrigde University Press; 2007: 45-82. [ Links ]
4. JANKOVIC J, ADLER CH, CHARLES D, COMELLA C, STACY M, SCHWARTZ M, SUTCH SM, BRIN MF, PAPAPETROPOULOS S. Rationale and design of a prospective study: Cervical Dystonia Patient Registry for Observation of On a Botulinum toxin A Efficacy (CD PROBE). Neurology 2011; 11: 140. http://www.biomedcentral.com/147-2377/11/140. [ Links ]
5. SEKEFF-SALLEM FA, CARAMELLI P, BARBOSA ER. Cross - cultural adaptation of the Toronto Western Spasmodi Torticollis Rating Scale (TWSTRS) to Brazilian Portuguese. Arq. Neuro-Psiquiatr 2011; 69(2B): 316-9. [ Links ]
6. MÜLLER J, KEMMLER G, WISSEL J, SCHNEIDER A, VOLLER B, GROSSMANN J, ET AL. The impact of blepharospasm and cervical dystonia on health related quality of life and depression. J Neurol 2002; 429: 842-846. [ Links ]
7. BEN - SHOLMO Y, CAMFIELD L, WARNER T. What are the determinants of quality of life in people with cervical dystonia? J Neurol Neurosurg Psychiatry 2002; 72: 608-614. [ Links ]
8. LEW MF, ADORNATO BT, DUANE DD, DYKSTRA DD, FACTOR SA, MASSEY JM, ET AL. Botulinum toxin type B: A double - blind, placebo - controlled, safety and efficacy study in cervical dystonia. Neurology 1997; 49: 701-707. [ Links ]
9. COSTA J, ESPIRITO - SANTO CC, BORGES AA, MOORE P, FERREIRA J, COELHO MM, SAMPAIO C. Botulinum toxin type A versus anticholinergics for cervical dystonia. Cochrane Database of Systematic Reviews 2005. Issue 1. Art. No.: CD 004312. [ Links ]
10. BRANS JW, LINDEBOOM R, SNOEK JW, ZWARTS MJ, VAN WEERDEN TW, BRUNT ER, VAN HILTEN JJ, VAN DER KAMP W, PRINS MH, SPEELMAN JD. Botulinum toxin versus trihexyphenidyl in cervical dystonia: a prospective, randomized, double - blind controlled trial. Neurology 1996; 46: 1066-72. [ Links ]
11. COSTA J, ESPIRITO-SANTO CC, BORGES AA, FERREIRA J, COELHO MM, MOORE P, SAMPAIO C. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database of Systematic Reviews 2005. Issue 1. Art. No.: CD 003633. [ Links ]
12. BRIN MF, LEW MF, ADLER CH, COMELLA CL, FACTOR SA, JANKOVIC J, ET AL. Safety and efficacy of Neurobloc (botulinum toxin type B) in type A - resistant cervical dystonia. Neurology 1999; 53: 1431-1438. [ Links ]
13. NAUMANN N, CARRUTHERS A, AURORA SK, ZAFONTE R, ABU-SHAKRA S, BOODHOO T, MILLER-MESSANA MA, DEMOS G, JAMES L, BEDDINGFIELD F, VANDENBURGH A, CHAPMAN MA, BRIN MF. Meta-analysis of neutralizing antibody conversion with on a botulinum toxin A (Botox) across multiple indications. Mov Disord 2010; 25(13): 2211-8. [ Links ]
14. COSTA J, ESPIRITO-SANTO CC, BORGES AA, FERREIRA J, COELHO MM, MOORE P, SAMPAIO C. Botulinum toxin type B in cervical dystonia. Cochrane Database of Systematic Reviews 2004. Issue 4. Art. No.: CD 004315. [ Links ]
15. FACTOR SA, MOLHO ES, EVANS S, FEUSTEL PJ. Efficacy and safety of repeated doses of botulinum toxin type B in type A resistant and responsive cervical dystonia. Mov Disord 2005 Sep; 20(9): 1152-60. [ Links ]
16. COSTA J, BORGES AA, ESPIRITO - SANTO CC, FERREIRA J, COELHO MM, MOORE P, SAMPAIO C. Botulinum toxin type A versus Botulinum toxin type B for cervical dystonia. Cochrane Database of Systematic Reviews 2003. Issue 3. Art. No.: CD 004314. [ Links ]
17. PAPPERT EJ, GERMANSON T. Botulinum Toxin Type B vs. Type A in Toxin Naïve Patients with Cervical Dystonia: Randomized, Double - Blind, Noninferiority Trial. Movement Disorders 2008; 23: 510-517. [ Links ]
18. COMELLA CL, JANKOVIC J, SHANNON KM, TSUI J, SWENSON M, LEURGANS S, FAN W. Comparison of botulinum toxin serotypes A and B for the treatment of cervical dystonia. Neurology 2005; 65: 1423-1429. [ Links ]
19. BRASHEAR A, LEW MF, DYKSTRA DD, COMELLA CL, FACTOR SA, RODNITZKY RL, TRPSCH R, SINGER C, BRIN MF, MURRAY JJ, WALLACE JD, WILLMER - HULME A, KOLLER M. Safety and efficacy of Neurobloc (botulinum toxin type B) in type A - responsive cervical dystonia. Neurology 1999; 53: 1439-1446. [ Links ]













