INTRODUCTION
Multiple sclerosis (MS) is an autoimmune, inflammatory, demyelinating, and chronic disease of the central nervous system (CNS), with subsequent neuro-axonal damage 1. Clinically it is characterized by attacks of dysfunction or organic malfunctioning of the CNS in its early stages, and by progressively worsening neurological deterioration in later stages. The relapses (or flares) are defined as the emergence of neurological symptoms that last more than 24 hours with no fever 2.
Worldwide, MS affects more than 2 million people and at least 400,000 in the United States 3. In Colombia, for the year 2000, a prevalence ranging from 1.48 to 4.89 for every 100,000 inhabitants was estimated 4-7. According to the study conducted by Perez-Jiménez et al. 3,462 patients (70% women) diagnosed with MS were treated in the country between 2009 and 2013, with an estimated prevalence of 7.52 per 100,000 inhabitants. The regions of the country with the highest prevalence of this disease are Bogotá (16.25 per 100,000 inhabitants), Quindío (13.3 per 100,000 inhabitants) and Risaralda (11.18 per 100,000 inhabitants) 8.
Natalizumab is a humanized monoclonal antibody that specifically binds to the α4 subunit of α4-[31 integrin, which prevents white blood cell migration in the brain and reduces inflammation 9. Therefore, the mechanism of action of natalizumab is mainly preventing white blood cells from crossing the blood-brain barrier, thus decreasing local inflammation of the CNS. Natalizumab is a highly effective therapy for relapsing-remitting multiple sclerosis (RRMS) 10; however, treatment with natalizumab has been associated with a risk of developing progressive multifocal leukoencephalopathy (PML) 10.
NEDA ("no evidence of disease activity") is defined as a treatment goal in which the absence of obvious disease activity is achieved. NEDA status is a composite outcome comprised of three related measurements of disease activity: no relapses, no disability progression, and no MRI activity (new or enlarging T2- lesions and gadolinium-enhancing lesions) 11.
The annual cost of multiple sclerosis for the United States' healthcare system is approximately 10 trillion dollars 12. In Latin America, treatment of MS is also expensive; countries such as Brazil, Argentina, and Colombia classify MS as a high-cost disease. Treatment costs vary according to the stage of the disease and the patient characteristics, ranging from US $6,511 to US$77,938 per patient-year 13. Therefore, despite its relatively low prevalence, MS generates a relatively high amount of healthcare expenses 14.
The costs associated with MS are generated mainly by disease-modifying drugs or derived from relapses, such as hospital stays, imaging, and laboratory tests. This analysis aims to provide a description of a group of MS patients treated with natalizumab to contribute to providing local information about the disease.
METHODOLOGY
The clinical records of 22 patients with MS who were followed for a mean of 9.2 years (range: 1.9-18.3 years) between 2000 and 2018 were analyzed. These patients received treatment with natalizumab in a high-complexity neurological outpatient clinic in Bogotá, Colombia.
A descriptive analysis of the information was performed to identify the main characteristics of the disease in the study population. The source of information was the patients' clinical records. Data were recorded in an Excel file by an expert neurologist who followed these patients (JRGB).
RESULTS
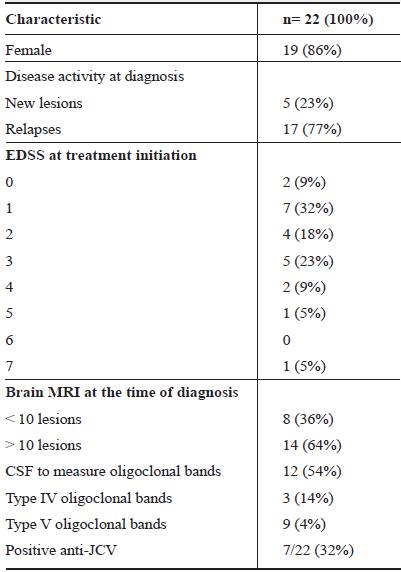
Table 1 shows a summary of the main clinical characteristics of the patients; 19(86%) were women. The sample included patients treated between 2000 and 2018. In 2000, two diagnoses were made, which account for 9% of the sample studied. From 2009 to 2010, there were six new diagnoses (17%), which were followed for 12 years (17%). Of the 22 patients, only 1 (5%) received natalizumab as initial treatment; 8 (36%) started treatment with interferon β -1a; 7 (32%) interferon β - b; 1 received interferon β -1a + β-1b; and 2 (9%) received fingolimod.
In 9 patients the same EDSS after follow up was maintained, in 4 patients it worsened (EDSS 3 to 5, in one, 2 to 3 in one, and 0 to 1 in the other two). The other 9 patients (50%) had improvement in their EDSS score: a 1 point improvement in four of them, two had a 2 point improvement, and three a 3 point improvement.
Anti-JC virus antibodies: The association between levels of anti-JCV antibodies in the serum or plasma and the risk of PML was assessed in all the patients. The measurement of anti-JCV antibodies was carried out using Enzyme-Linked Immunosorbent Assay (ELISA), with an optical density (OD) cut-off point of ≤0.40 15. Seven patients (32%) had positive titers of anti-JCV antibodies and three patients (14%) had titers lower than 0.40 (negative). None of them developed PML. Furthermore, none of these patients met the risk criteria for developing PML, that is, positive anti-JCV status before natalizumab treatment and the previous use of immunosuppressant drugs before treatment with natalizumab 16.
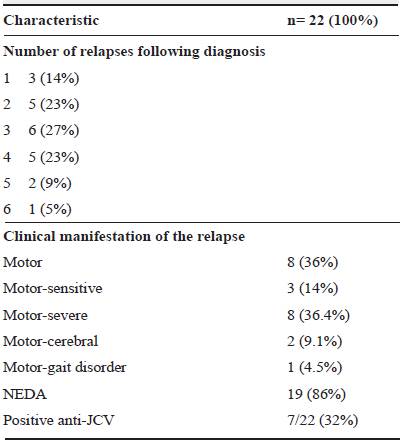
Five patients had disease activity related to the presence of new lesions (23%) and 17 patients (77%) had disease activity due to relapses (Table 1). The clinical manifestation of relapses was mainly motor impairment; 8 patients (36%) developed motor impairment (relapse) in the first year and one 13 months after the diagnosis.
The definition of NEDA status is strictly related to clinical practice and there is evidence to support the recommendation of actively treating MS using NEDA status as a treatment goal. NEDA-4 refers to a status in which brain atrophy or presence of cerebrospinal fluid neurofilament light chain are included; NEDA-4 refers to when there is no brain atrophy, and NEDA-5 when levels of cerebrospinal fluid neurofilament light chain are not measurable 11.
NEDA status was evaluated in December 2017 and April 2018 in 19 patients (86%) (table 2). The median time in which patients reached NEDA status was six months (IQR= 3-6).
Eight patients initially treated with interferon β-1a (36%) had therapeutic failure, because of which treatment was escalated to natalizumab. One of these patients had to switch to fingolimod (the third line therapy for this patient) since natalizumab treatment had no therapeutic effectiveness. However, this patient could not achieve NEDA status with ingolimod.
Of the seven patients treated with interferon β -1b as first line treatment (32%), four developed therapeutic failure, so treatment was escalated to natalizumab and, for one patient, to fingolimod before moving to natalizumab. The remaining three patients of this group had disease activity, so they were later escalated to natalizumab. Only one patient still displays disease activity, while the rest have reached NEDA status.
One subject who received combined treatment with interferon β-1a and interferon β-1b at the beginning was escalated to fingolimod due to relapses; however, because of lesion progression, management with natalizumab was eventually initiated. An appropriate clinical course and therapeutic response were achieved with this treatment without adverse reactions to the drug, and NEDA status was achieved.
Finally, two subjects (9%) received fingolimod as first line treatment. However, following the presence of severe headache and relapse, treatment was changed to natalizumab in both cases. NEDA status was then achieved with this treatment and no serious adverse effects were observed.
DISCUSSION
Natalizumab is a highly effective and safe therapy for RRMS, as shown in clinical trials by Polman and Rudick 17,18. Moreover, evidence has shown its clinical efficacy compared with other drugs available for MS treatment, such as fingolimod and interferon 19-22.
Currently, NEDA status and prevention of potential damage to vital organs have become the goal of MS treatment, and the challenge is to stop disability progression in later stages of the disease. In clinical practice, it is also considered that, to achieve NEDA, it is advisable to re-assess patients periodically after the start of disease-modifying therapies to follow treatment response closely as well as possible adverse reactions 11. In this analysis more than 80% of the patients reached NEDA status, -which is one of the treatment goals- with natalizumab. Furthermore, patients with natalizumab and NEDA-4 status did not have evidence of clinical disease activity or cerebral atrophy, which is consistent with earlier findings reported in the literature 11. Also, in this case series most patients had an improved EDSS score.
In this analysis, natalizumab showed consistent effectiveness and a good safety profile, similar to the results from the Safety of TYSABRI Re-dosing and Treatment (STRATA) Study conducted by O'Connor et al., which evaluated 1,094 patients from previously conducted natalizumab clinical trials comparing the effectiveness of natalizumab with placebo. In this study, patients randomized to placebo had higher EDSS scores compared to natalizumab (3.13 vs 2.90; p = 0.027), while placebo and natalizumab groups had similar mean EDSS scores at the beginning of the studies (2.36 vs 2.38), EDSS scores for those randomized to placebo worsened during the study period (2.69 vs 2.36). At week 240 of follow-up, mean EDSS scores remained stable in both groups (3.15 vs 2.79; p = 0.024) 9.
Patients originally randomized to natalizumab had a lower annualized relapse rate compared to patients originally randomized to placebo (0.15 vs 0.22), however a reduction beyond 48 weeks was seen in both groups. The statistical difference between groups was noted both during the first year (p < 0.01) as well as during the overall study period (p < 0.01) 9.
In the same study, 171 patients (16%) had at least one serious adverse event (excluding MS relapse). No single serious adverse event was seen in more than 2% of patients. The most common serious adverse events were infections and infestations, gastrointestinal disorders, and neoplasms 9.
Treatment with natalizumab has been associated with the risk of developing PML 10. Bloomgren et al. reported the incidence of PML related to natalizumab in 2.1 cases per 1000 patients, also determining that risk of PML is lower among patients with negative anti-JCV antibodies, for whom the incidence is estimated to be 0.09 cases or less per 1000 patients (95% CI, 0-0.48) 16. On the other hand, the incidence among patients with positive anti-JCV antibodies who took immunosuppressant drugs for 25 to 48 months before the start of natalizumab treatment recorded the highest risk of developing such a complication, with an incidence of 11.1 cases per 1000 patients (95% CI, 8.3-14.5) 16.
By controlling these risk factors during treatment with natalizumab, the incidence of PML can be decreased using good clinical practices, personalized treatment, and active follow-up during the course of the disease. In our patients risk factors were monitored during the follow-up period through the identification of positive anti-JCV antibodies, previous use of immunosuppressant drugs and duration of natalizumab treatment 10,16. Despite seven patients with positive anti-JCV antibodies, none of them developed PML up to the last point of analysis.
In Europe, the costs of neurological diseases for 2010 was estimated at 798 trillion Euros per year, with an average cost per inhabitant of 5,550 Euros 14. The high costs of MS for the health system are also reported in countries such as Argentina and Brazil; likewise, MS is a high-cost disease in Colombia 13. There are few studies in Colombia about the costs of multiple sclerosis. A recent study (2018) carried out by Munoz-Galindo and collaborators 23 evaluated a cohort of multiple sclerosis patients, to find out the associated costs of MS for the health system, from the perspective of a private payer 23. The annual average cost per patient in Colombia (in US dollars, USD) was USD $ 29,340 in 2010, USD $ 20,956 in 2011, USD$ 23,893 in 2012, USD$ 24,148 in 2013, and USD$ 22,688 per patient in 2014.
CONCLUSIONS
This study gave an exploratory analysis of the characteristics of a series of patients with MS in the Colombian context. In the retrospective analysis of this patients, it was observed that more than 80% of the studied sample that received treatment with natalizumab, reached NEDA status. Despite the methodological limitations inherent to the type of study and sample size, this study suggests that natalizumab could be an appropriate medication for the management of MS in Colombia. Given the findings of this study, it is imperative that research in this field continues, to identify more effective and safe therapeutic strategies for the management of MS.